
Chemistry
- Matter, Kinetic Theory
- Compounds, Mixtures & Separation
- The Atom
- Stoichiometry & Reactions
- Chemical Bonding
- Electrolysis
- Water & Solutions
- Rates of a Reaction
- Enthalpy
- Gases & Gas Laws
- Acids, Bases & Salts
- Non-metals and Compounds
- Organic Chemistry I
- Organic Chemistry II
- Metals & Compounds
- New Tab
- New Tab
MATTER, KINETIC THEORY
Matter is anything that has weight and occupies space.
It exists in three physical states namely solid, liquid and gas. For example, water can exist as ice (solid), water (liquid) and steam (gas)
A matter may be characterized by its
Chemical properties: refers to the chemical changes that matter undergoes. E.g rusting of iron
Physical properties (attributes that distinguish it from other types of matter) such as density, colour, melting and boiling point, physical state, electrical conductivity, and so on.
When matter is uniform in composition and physical properties irrespective of the state, it is called a phase.
So if a material has only one phase it is said to be homogeneous e.g. iron, copper, salt, a solution of salt and water.
| Physical | Chemical |
| It is easily reversible e.g. the gas during vaporization of a liquid can easily be condensed to get the liquid | It is not easily reversible e.g. burning of substances |
| It produces no new substances e.g. melting of solid to a liquid | New substances are formed. E.g rusting of iron |
| It is not accompanied by large heat changes (except in the case of latent heat when matter changes state) | A considerable amount of heat change is involved e.g. exploration of coal gas with hydrogen with air |
Kinetic Theory of matter
This simply says the molecules that make up matter are in onstant motion (irrespective of the state they are). We can thus describe the characteristics of the three states of matter from the perspective of molecules
Characteristics of the solids, liquids and gases
Solid state
- The molecules, atoms or ions that make up a solid are closely packed and held together by strong cohesive forces (force of attraction between molecules of the same substance).
- These forces of cohesion may come from electrovalent, covalent, metallic or even van der Waals bonds.
- The particles can only vibrate or rotate about a fixed position
- They have definite shape and volume
- They are difficult to compress
Liquids
- The particles are slightly further apart than those of a solid.
- The particles can vibrate, rotate and translate and are no longer held in fixed positions. Thus, the particles of a liquid can slide over one another and it pours.
- They have fixed volume but no definite shape or form. They assume the shape of their container in which it is placed
- They are difficult to compress
Gases
- The particles have much more kinetic energy than those of a solid or liquid.
- The cohesive forces between gas particles are negligible and they are free to move about in all directions.
- A gas has no definite shape or size and they occupy the whole volume of the container
- They can be easily compressed
Change of state
A change of state (between solid, liquid and gas) is brought about by a temperature change, that is, heating or cooling.
Explanation using kinetic Theory
Melting
When a solid is being heated, the molecules absorb the heat energy and they begin to vibrate vigorously. These vibrations soon become energetic enough to break the attractive forces with surrounding molecules and they begin to slide out of their position to flow about. The solid is now melting into a liquid.
Evaporation
When the particles of a liquid are moving with different velocities, the faster ones at the surface acquire enough kinetic energy to break away from the attraction of other molecules and then escape from the surface of the liquid. This is evaporation.
The average kinetic energy of the remaining molecules then decrease leading to a reduction in temperature. Thus evaporation brings cooling. This is why you feel cool when sweat evaporates from your skin
Boiling
When a liquid is heated, the molecules acquire kinetic energy and they move faster as the temperature rises. A point is reached when the heat energy absorbed to overcome the attraction forces does not cause a temperature change. The molecules now have negligible forces of attraction. The liquid has boiled off to become a gas
Freezing
When heat is gradually removed from a liquid, the molecules become slow and can be held together by the forces of attraction, thus becoming a solid
Condensation
Vapour pressure
Evidence for kinetic Theory
Brownian movement
Diffusion: This the movement of molecules from a region of higher concentration to a region of lower concentration.
Osmosis: It is the movement of water molecules from a region of higher concentration to a region of lower concentration through a semi-permeable membrane
.
ELEMENTS, COMPOUNDS & MIXTURES
Element and compounds are pure substances.
An element is a substance that cannot be broken down into simpler substances. E.g. sodium, oxygen
There are over 118 known elements arranged in the periodic table according to their atomic numbers. These elements are grouped into metals, non-metals and metalloids (semi-metals)
A compound consists of two or more elements in a fixed proportion chemically combined together. E.g. water, ammonia
A mixture is a substance formed when two or more constituents are physically combined together. E.g. air, urine, blood, crude oil
| Mixture | Constituents |
| Air | Nitrogen, oxygen, carbon (IV) oxide |
| Urine | Urea, water, mineral salts |
| Blood | Water, proteins, hormones, enzymes, haemoglobin |
| Crude oil | Petrol, diesel, kerosene, bitumen |
| Bronze | Copper, tin |
Differences between compounds and mixtures
| Compounds | Mixtures |
| It is always homogeneous | It can be homogeneous or heterogeneous |
| The elements are chemically bound together and cannot easily be separated | The constituents can easily be separated by physical means |
| It can be represented by a chemical formula because the elements are present in a fixed ratio by mass | It cannot be represented by a chemical formula |
| The properties of a compound are different from those of the component elements. E.g. Sodium conducts electricity but sodium chloride is a poor conductor (except in molten state) | The properties of a mixture are a sum total of the properties of the individual constituents |
Separation Techniques
Mixtures can be separated using the following methods
- Sieving
- Decantation
- Filtration
- Distillation
- Fractional Distillation
- Precipitation
- Sublimation
- Crystallization
- Chromatography
Sieving
It is used to separate a mixture of solid particles of different sizes.
The mixture is placed on a sieve with a mesh of a particular size.
Particles smaller than the size of the mesh will pass through, while the larger particles will remain
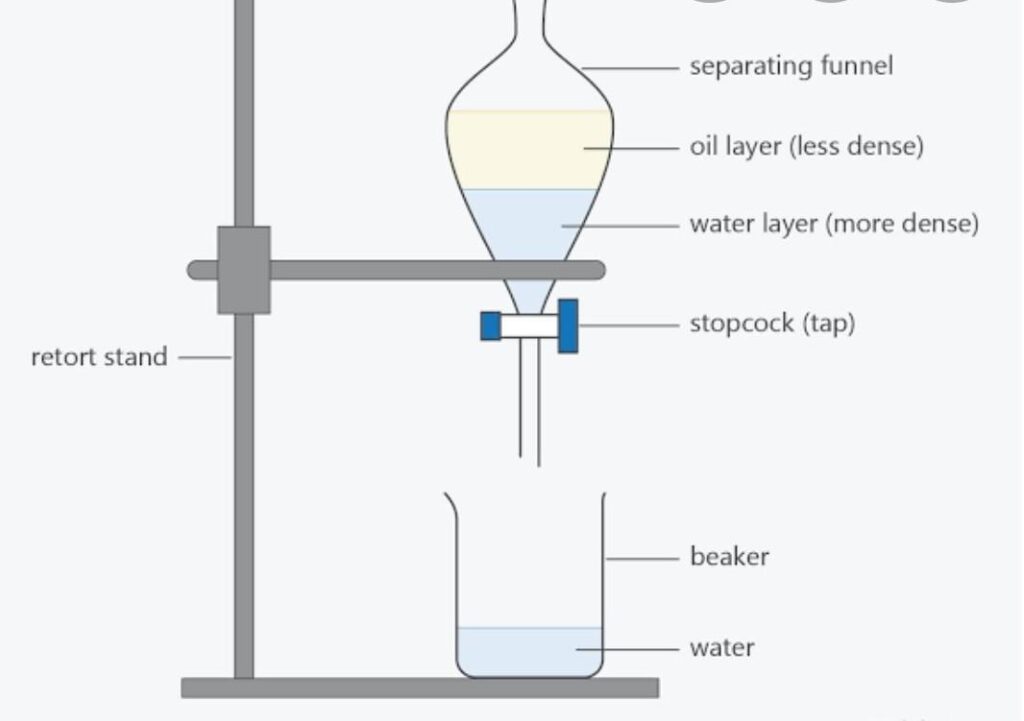
Separating Funnel/Decantation
This is used to separate a mixture of a liquid and denser solid particles.
This mixture separates into two distinct layers on standing.
The upper layer of the clear liquid is carefully poured out into another container leaving the solid portion at the bottom.
Would you call this method accurate?
Filtration
This is a method of separating a liquid from an insoluble solid. E.g sand and water.
During filtration, the liquid portion which passes through the filter paper is called the filtrate while the portion that remains on the filter paper is the residue.
Filtration is extensively used in water purification

Distillation
This is a process used to separate a mixture of two miscible liquids whose boiling points are wide apart. For example, a mixture of alcohol (which boils at 78 degrees celsius) with water (boils at 100 degrees celsius) can be separated by distillation.
The solution is heated in a flask to vapourize the more volatile liquid. The vapour passes through a condenser in order to turn it back into a liquid. Distillation is used in breweries and gin distilleries to purify liquids
Fractional Distillation
A more accurate form of distillation is fractional distillation.
It is used to separate a mixture of two or more liquids with close boiling points into its component fractions.
The apparatus in fractional distillation is the same as in simple distillation except that a fractionating column is introduced between the distillation flask and the condenser.
Fractional distillation is used to separate petroleum into its many important components such as petrol, diesel, kerosene, natural gas, bitumen, etc. It is also used to separate liquefied air into nitrogen, oxygen and noble gases.
Evaporation
In this method, we are separating a soluble solid from a liquid by heating the mixture to dryness.
Evaporation is the process of vapourization of a liquid at all temperatures.
The liquid portion with a lower vapour pressure than the solid is removed from the solid portion. E.g. salt solution.
The solvent (water in salt solution) is sacrificed in this process. This process is used in salt-making industries
Crystallization
This is used to separate salts (which easily decompose on heating) from their solutions.
It is used in the drug manufacturing industries where purity is important
Sublimation
Some solids when heated change directly to gas without passing through the liquid state.
Such physical change is called sublimation.
Examples of solids that sublime include ammonium chloride, iodine, sulphur.
Also camphor, solid air-freshner sublime.
Thus we can separate substances that sublime from others e.g. chalk and camphor by simply heating.
The substance that sublimes is sometimes sacrificed (or can be recovered by adding some control measures)
Precipitation
This chemical process uses the differences in solubilities of solids in different liquids. For example, iron (III) chloride is soluble in water but insoluble in ethanol.
So adding ethanol in such a solution will leave a precipitate which can be filtered and recrystallized
Chromatography
This is a method of separating the constituents of a mixture based on their different speeds in a solvent over an absorbent medium.
Chromatography is used to separate the dyes in coloured ink. It is used in medicine to analyse blood or urine.
Centrifugation
Pure and Impure Substances
THE ATOM
An atom is the smallest particle of an element that can take part in a chemical reaction.
An element in nature can be
monoatomic, e.g. Na, Mg, Ne
Diatomic, O2, N2, Cl2, H2
Triatomic: ozone (O3)
Structure of the Atom
Different scientists over the years have tried to explain the structure of the atom.
In 1808, John Dalton put forward his famous atomic theory based on chemical laws and experimentation known at that time. Even though the theory is still basically valid today, some modifications have been made in light of modern discovery (such as the electron microscope).
Dalton: All elements are made of up small indivisible particles called atoms
Modification: Rutherford’s alpha scattering experiment showed that an atom is made of three main subatomic particles namely protons, electrons and neutrons
Dalton: atoms can neither be created nor destroyed
Modification: this is agreed for ordinary chemical reactions. In nuclear reactions however, the changes that occur in nuclear fission tend to destroy atoms of the elements involved
Dalton: atoms of the same element are exactly alike in every aspect and are different from atom of other elements
Modification: the discovery of isotopes proves this statement wrong. Chlorine has two stable isotopes namely, chlorine-35 and chlorine-37. Both have 17 protons and 17 electrons but different neutrons. Chlorine-37 has 20 neuttrons while clorine-35 has 18 neutrons. Thus, chlorine-37 is heavier
Dalton: atoms of different elements combine in simple whole number ratios to form compound
Modification: this is acceptable for inorganic compounds where few atoms per molecule are obtainable. Carbon, however, forms very large compounds such as in proteins, starch and fats which contains thousands of atoms
J. J. Thompson suggested the plum-pudding model where the atom is a ball of positive charges with negative electrons embedded in it
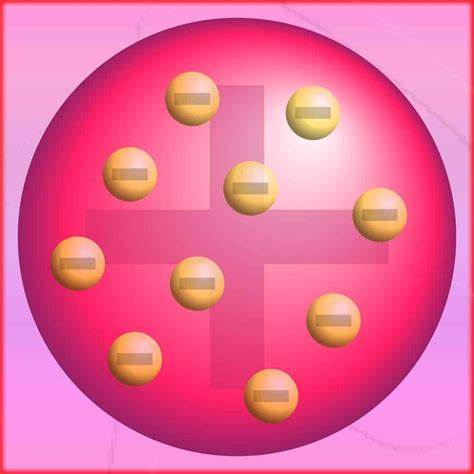
Lord Rutherford Model suggested a nuclear model that consists of a nucleus which contains most of the positive charges with the electrons moving in orbits
Neil Bohr said the electrons orbit the nucleus in shells
James Chadwick discovered particles in the nucleus which had no charge – the neutrons
Atomic Number and Mass number
Atoms consists of protons, neutrons and electrons.
The atomic number of an element is the number of protons present in the nucleus of the element. It is denoted by the letter Z.
For a neutral atom, the number of protons is equal to the number of electrons. Thus, the atomic number is also equal to the number of electrons. The atomic number determines the nature of the atom.
The protons and neutrons are found in the nucleus which contains most of the mass of the atom and all of the positive charge. The neutrons help to reduce repulsion between the positively charged protons.
The electrons are arranged in energy levels (shells) around the nucleus and it is this arrangement that determines the chemical properties of the element
The mass number of an element is the sum of the protons and neutrons in the atom. It is denoted by the letter A. For example, flourine has a mass number of 19.
we can now describe the atom of an element by writing the symbol of the element, the atomic number and mass number.
ZAX as in 1123Na or 2040Ca
atomic number = number of protons
mass number = number of protons + number of neutrons
number of electrons = number of protons in a neutral atom
number of neutrons = mass number – atomic number/protons
Isotopes
Isotopes are atoms with the same atomic number but different mass numbers
For example, chlorine has two isotopes – chlorine-35 and chlorine-37.
Both have 17 protons. However, chlorine-35 has 18 neutrons, while chlorine-37 has 20 neutrons.
Isotopes of an element have the same chemical properties because they have the same electron arrangement but may have different physical properties such as rate of diffusion since they have different masses.
Ions
Ions are formed when atoms gain or lose electrons
The chlorine atoms, Cl, has 17 electrons. When it gains an electron, it forms the chloride ion, Cl–, which now has 18 electrons. Sodium ion, Na+, with 10 electrons is formed when the sodium atom, Na, having 11 electrons loses one electron.
Early models of atomic structure predicted that atoms and ions with noble gas structure electron arrangements should be stable.
Cl– has the same electron arrangement as Argon, while Na+ has the same arrangement as neon.
Molecule
A moelcule is the smallest particle of a substance which cannot be broken further. A molecule could be an element or a compound.
For example, O means 1 atom of oxygen, O2 means 1 molecule of oxygen or 2 atoms of oxygen. NH3 means 1 molecule of ammonia; H2O means 1 molecule of water. Thus, in the equation balanced equation
2H2(g) + O2(g) –> 2H2O(g)
2 molecules of hydrogen gas (4 atoms of hydrogen) reacts with 1 molecule of oxygen gas (2 atoms of oxygen) to give 2 molecules of water
Relative Atomic Mass
In the early days, scientists could not measure the mass of one atom directly because it was too small. They had to compare the mass of one of an element with the mass of the hydrogen atom – the lightest known atom.
The hydrogen was assigned the basic mass value of 1 and based on this, they found for example that the relative atomic mass of oxygen is 16 times that of hydrogen, while that of sodium is 23 times that of hydrogen.
Relative atomic mass carries no units as it is only a ratio.
Today, the mass of atoms can be measured very accurately using a mass spectrometer. And we find that the mass of the hydrogen atom is 1.67 x 10-24g while that of oxygen is 2.66 x 10-23g. Now, since these masses are too small to be practically useful, the relative atomic mass values are still being used. However, modern scientists have changed the base mass from hydrogen to carbon-12 since the latter is stable and abundant in nature.
Relative Atomic mass (Ar) of an element is the number of times the average mass of one atom of that element is heavier than 1/12 of the mass of one atomo f carbon-12
relative atomic mass = (average mass of 1 atom)/1/12 mass of 1 atom of carbon-12)
Relative molecular mass (Mr) of an element or a compound is the numbe ro ftimes the average mass of on emolecule of it is heavier than one-twelfth of the mass of one atom of carbon-12.
For example, calculate the relative molecular mass of limestone, CaCO3 given the following relative atomic masses: Ca = 40, C = 12, O = 16
Mr = (1 x 40) + (1 x 12) + (3 x 16)
=40 + 12 + 48
=100
Mole
One mole of a substance is the amount of that substance that has the same number of specific particles (atoms, molecules or ions) as there are atoms in exactly 12g of carbon-12.
The mole (mol for short) is a quantitative unit that scales up the amount of a substance so that it becomes easy to weigh. Working with atoms or molecules is impracticable because they are extremely small. Thus, a mole of sodium (Ar = 23) weighs 23.0g
Molar Mass
This is the mass of 1 mole of a substance
STOICHIOMETRY & REACTIONS
Mole Calculations
We can find the number of moles of a substance by using the mass of substance and the molar mass
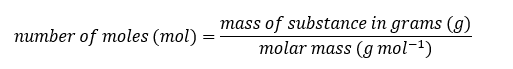
Example: How many moles of sodium chloride are present in 117.0 g of sodium chloride, NaCl?
[Na = 23, Cl = 35.5]
Percentage Composition by mass
The sum of the masses of the moles of component elements in a compound will give the mass of 1 mole of the compound
For example,
Mass of 1 mole of CaCl2 = mass of 1 mole of Ca atoms + mass of 2 moles of chlorine atoms
=40 g+71 g
111 g
We can also calculate the percentage by mass of a particular element in a compound using the formula
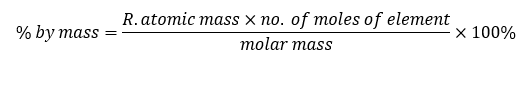
Other Examples
Calculate the percentage by mass of Sulphur in tetraoxosulphate (VI) acid, H2 SO4
[H = 1, S = 32, O = 16]
Calculate the percentage by mass of all the component elements in sodium trioxonitrate (V) NaNO2
[Na = 23, N = 14, O = 16]
Calculate the percentage by mass of iron in iron (III) oxide, Fe2 O3.
[Fe = 55.8, O = 16]
Empirical and Molecular Formula
The empirical formula of a compound is the simplest whole number ratio of the elements present in one molecule of the compound.
The molecular formula of a compound shows the total number of atoms of each element present in molecule
| Compound | Empirical formla | Molecular formula |
| Water | H2O | H2O |
| Hydrogen peroxide | HO | H2 O2 |
| Butane | C2 H5 | C4 H10 |
| Ethyne | CH | C2 H2 |
| Benzene | CH | C6 H6 |
The empirical formula can be found by determining the mass of each element present in a sample of the compound.
It is important for the compound to be pure in order to calculate its empirical formula. Chemists often use gas chromatography to purify the compounds before carrying out the analysis
To deduce the formula, find the mole ratio of each component element in the compound from
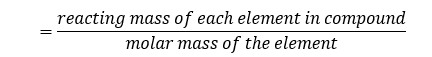
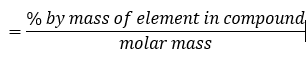
A compound of carbon, hydrogen and chlorine contains 0.48 g of carbon, 0.08 g of hydrogen and 1.42 g of chlorine.
(i)Determine the empirical formula of the compound
(ii)If the molar mass of the compound is 99, calculate the molecular formula of the compound. [H = 1.0, C = 12.0, Cl = 35.5] (WASSCE’ 18)
Solution
Mole ratio:
C:0.48/12.0=0.04, H:0.08/1.0=0.08, Cl:1.42/35.5=0.04
Divide by the smallest value
0.04/0.04=1 0.08/0.04=2, 0.04/0.04=1
Empirical formula is CH2 Cl
Since molar mass is 99,
(CH2 Cl)x=99
(12+2+35.5)x=99
49.5x=99
x=99/49.5=2 molecular formula is (CH2 Cl)2 or C2 H4 Cl2
Mass-mass relationships in chemical reactions
When substances react together, we may need to know what mass of each reactant to use so that they react exactly without any waste.
To calculate this, we require the balanced equation of the reaction as it tells us the stoichiometry of the reaction, that is, the ratio of moles of the reactants and products
Consider the reaction
Fe2 O3+3CO→2Fe+3CO2
1 mole of iron (III) oxide reacts with 3 moles of carbon (II) oxide to form 2 moles of iron and 3 moles of carbon (IV) oxide.
The stoichiometry of the reaction is 1:3:2:3
Now, let’s put it in a practical example
Calculate the maximum mass of iron produced when 1596 g of Iron (III) oxide reacts with carbon (II) oxide to form iron and carbon (IV) oxide.
Fe2 O3+3CO→2Fe+3CO2
[Fe = 55.8, O = 16]
Solution
The mole ratio is 1:3:2:3
1 mole of Fe2 O3 produces 2 moles of iron
From No. of moles=mass/(molar mass),
For Fe2 O3, 1=mass/(2×55.8+3×16)
mass=159.6 g
For Fe, mass=No. of moles×molar mass=2×(55.8)=111.6 g
So 159.6g of Fe2 O3 produces 111.6 g of Fe
∴1596 g of Fe2 O3 will produce x g of Fe
159.6×x=1596×111.6
x=(1596×111.6)/159.6=1116g
Laws of Chemical Combination
These 4 laws describe the general features of a chemical change
1.Law of conservation of mass
It states that matter is neither created nor destroyed during a chemical reaction but changes from one form to another
2. Law of definite proportions
It states all pure samples of a particular chemical compound contain similar elements combined in the same proportion by mass
If we consider water as an example, we see that as it is pure, its composition will always be 2 moles of hydrogen molecules to 1 mole of oxygen molecules or 4g to 32g
3. Law od multiple Proportions
It states that if two elements A and B combine to form more than one compound, then the various masses of A which combines separately with a fixed mass of B, are in simple multiple ratio.
For example
Iron and chlorine react to give iron (III) chloride, FeCl2, and iron (II) chloride, FeCl2
For example
Iron and chlorine react to give iron (III) chloride, FeCl3, and iron (II) chloride, FeCl2
| Compounds | Iron (II) chloride | Iron (III) chloride |
| Moles of iron atoms (fixed) | 1 | 1 |
| Moles of chlorine atom (variable) | 2 | 3 |
| Ratio of chlorine masses in the chlorides of iron | 2 | 3 |
Thus we see that the multiple masses of chlorine that combines with a fixed mass of iron are in simple ratio 2: 3
4. Law of Reciprocal Proportions
It states that the masses of several elements A,B, C which combine separately with a fixed mass of another element, D, are the same as or simple multiples of, the masses in which A, B, C themselves combine with one another
Balancing Chemical Equations
In a chemical reaction, the reactants are written in their formula form on the left-hand side (LHS) of the equation while the products are written on the right-hand side (RHS).
An arrow symbol (→) leads from the reactants to the products
For example,
2H2+O2→2H2 O
Where a chemical reaction cannot occur, it is wrong to write an equation to represent it.
To balance chemical equations,
•we apply the law of conservation of matter, which states that atoms can neither be created nor destroyed
•the formulae of the reactants and products are fixed and cannot be altered
•take appropriate number of moles of the reactants and product concerned
•do an atom count to check that the equation is balanced
WOrked Example
Balance the following chemical equations
CH4+O2→CO2+H2 O
NH3(g) +O2(g) →NO((g) )+H2 O((g) )
A balanced chemical equation tell us
The reactants and the products involved
The stoichiometry of the reaction, that is, the number of moles of the reactants and products required
The states of matter in which the reactants and products are present. This is indicated in the state symbols (s)-solid, (l)-liquid, (g)- gas, (aq)-aqueous
PRACTICE QUESTIONS
On analysis, 1.0g sample of a hydrocarbon was found to contain 0.923g of carbon. If the vapour density of the hydrocarbon is 39.0 (i) determine its molecular formula
(ii) name the compound
(i) Define relative atomic mass (ii) What phenomenon is exhibited by an element Z which exists as 3517Z and 3717Z (iii) What accounts for the difference in the mass numbers of the element Z (iv) Calculate the relative atomic mass of Z if the percentage abundance of 3517Z is 75%
Consider the reaction equation:
Fe + H2SO4 → FeSO + H2
calculate the mass of unreacted iron when 5.0 g of iron reacts with 10cm3 of 1.0 moldm-3 H2SO4
[Fe = 56.0]
CHEMICAL BONDING
Chemical Bonds
A chemical bond holds atoms or molecules together. These connections are essential to life because living things are made up of atoms. These atoms however are not just floating around individually but instead are usually interacting with other atoms or groups of atoms.
Why do atoms form bonds?
Atoms usually try to always reach the most stable state that they can. This is their lowest energy state. Thus many atoms become stable when this outermost shell is filled with electrons. A shell is filled when it satisfies the octet rule by having eight electrons in its outermost shell or duplet structure by having two electrons in the case of hydrogen, lithium, berylium.
Bonding occurs as a result of the different behaviours of the electrons in the outermost or valence shells.
To put it in another way: the valence electrons of atoms are responsible for the chemical properties of elements
Types of Bonding
There are two majors types of chemical bonds –
Electrovalent or ionic bonds
Covalent bonds
Electrovalent bonds
In this type of bonding, there is transfer of electrons from one atom (which is usually metallic) to another (usually non-metallic). We have already mentioned that the electrons involved are called valence because they reside in the outermost shell. Thus, we have the metallic atoms acting like electron donors and the non-metallic atoms acting like acceptors.
When atoms gain or lose electrons (to achieve stable duplet or octet structures), they form ions or charged particles.
Ions
There are two types of ions – cations which are positive ions and anions which are negative ions.
For example, a sodium atom has 11 protons and 11 electrons. Protons are positively charged while electrons are negatively charged. Based on the electronic configuration, it has 1 electron in its outermost shell. It loses this electron to become a sodium cation because there are now 11 protons and 10 electrons meaning the atom has more positively charged particles. We represent this sodium cation as Na+.
On the other hand, chlorine with atomic number 17, has 7 electrons in its valence shell. It is very much easier for chlorine to gain one electron (to form a stable octet) than lose seven, so chlorine after gaining one electron forms an anion represented as Cl-
When sodium and chlorine combine, sodium will donate its one electron to empty its shell from its outermost shell and chlorine will accept to fill its shell. Both have now achieved stable octet with complete eight electrons in their outermost shells. Sodium now has a charge of +1 while chlorine has a charge of –1.
Sodium Chloride, like many other ionic compounds, does not consist of just one sodium and chloride ion, that is, the oppositely chaged ions do not pair up to form molecules, instead, it contains many ions arranged in a repeating three-dimensional pattern, called a crystal.
Covalent Bonding
In this type of bonding, there is no transfer of electrons between atoms, instead, there is a sharing of a pair of electrons. Atoms can also achieve stable octet states by sharing electrons rather than donating or accepting them. This pair of electrons to be shared is called a shared pair. Each reacting atom contributes an electron to the shared pair.
These shared electrons may be regarded to be revolving round the nucleus of both nuclei in orbits. Thus, covalent bonds result in the fomation of small collection of connected atoms called molecules.
Sometimes, one, two or three pair of electrons may be shared resulting in single, double or triple covalent bonds. Covalent bonds are more common than ionic bonds in living organism.
Let’s look at the formation of water as an example of a covalent bond.
A single water molecule H20 consist of 2 atoms of hydrogen bonded to one atom of oxygen. Each Hydrogen atoms ahres an electron with oxygen and oxygen shares one of its electrons with each hydrogen
A covalent bond may be polar or non-polar. If the electrons are unequally shared by the contributing atoms, then is polar. This is the case of water molecule. Oxygen is amore electronegative atom than hydrgen; this means it will attract the shared electrons more strongly than the hydrogen atom. So the oxygen of water bears a partially negative charge (because it has higher electron density) while the hydrogen has a partial positive charge (because of a lower electron density.
No polar covalent bonds occurs between atoms of the same element or atoms of different elements but tend to have a fairly equal sharing power. For example, oxygen gas (O2) is non-polr because the electrons are equally shared between the two oxygen atoms equally. Methane CH4 is also said to non-polar
Coordinate Covalent Bonding
We have a special type of covalent bond called coordinate covelent bond. Here, the pair of electrons to be shared is contributed by only one participant. The pair of electrons here is described as a lone pair. Examples of this type of bond is the formation of the ammonium ion (NH4+) and the oxonium ion (H3O+).
When ammonia and hydrochloric acid reacts, the hydrogen ion from the acid accepts the lone pair of electrons from the ammonia molecule. This bond enables the hydrogen ion to acquire the stable duplet structure. The positive charge on the hydrogen ion is carried over to now give the positively charged ammonium ion
ELECTROLYSIS
Electrolysis is the breakdown of an electrolyte by the passage ofan electric current.
It is used to extract substances(especially reactive metals) from their ores. It is also used in electroplating.
An electrolyte is a solution that conducts electricity e.g. salt water, sulphuric acid. The charge carriers are the positive and negative ions which are free to move in the liquid.
Consider dilute sulphuric acid, the ions present
NOTE: an acid is a substance that produces hydrogen ions as its only positive ions when dissolved in water
H2SO4(aq) → 2H+ + SO42-
Components of electrolysis
•Anode: positive electrode
•Cathode: negative electrode
•Electrolyte
•DC source
An ion is an atom that has gained or lost an
electron. cation(+ve ion), anion (-ve ion)
During electrolysis, cations move
to the cathode, while anions move
to the anode
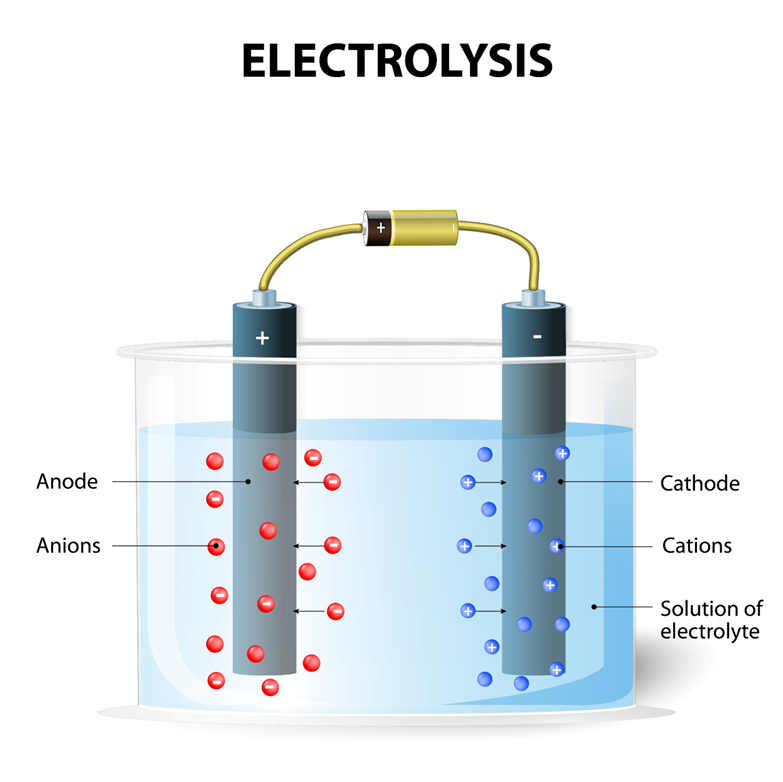
Electrolysis of dilute sulphuric acid
Ions in the mix
H+, SO42- ,
OH– (from the water)
H+ moves to the cathode
SO42- and OH– moves to the anode
‘Fear someone not clearly born in ohio’
F–, SO42-, NO3–, Cl–, Br–, I–, OH–
At the cathode: H+ + 1e→ H H+ +1e→H
2H+ + 2e→H2
H + H→H2
At the anode: 4OH– → O2 + 2H2O + 4e
2OH– + 2OH–→ 2OH + 2OH + 4e→ 2H2O+ O2+ 4e
Colourless gases are produced at each electrode. The volume of hydrogen produced at the cathode is twice the volume of oxygen produced at the anode
WATER & SOLUTIONS
Introduction
Water has to be the most common substance known to man. The chemical formula for water is H20
It consists of 2 atoms of hydrogen and 1 atom of oxygen
Sources of Water
We get water from rain, springs, lakes, wells, rivers and the sea. Rainwater is the purest form of natural water because it is formed from condensation of water vapour in the atmosphere.
Water from wells may require boiling before drinking
River, lake and sea water contain a lot of dissolved air, mineral salts, bacteria and organic remains. Special purification processes have to take place before they can be considered safe for drinking
Distilled water is chemically pure water. It can be prepared by condensing steam using a Liebig condenser.
It is used for preparing reagents in the laboratory
for reparation of drugs in pharmaceutical industries
in car batteries
Treatment of Water
Water from rivers, rainfalls or lakes should be purified before distributed to homes or industries through pipes.
The stages of purification can be summarized below
1. Coagulation and floculation
The untreated water is passed through large settling tanks where potash alum KAl(SO4 )2.12H2O or sodium aluminate NaAlO2 are added to cause the impurities to coalesce and form big particles which settle at the bottom of the tank.
2. Filtration
The water is now passed through a filter bed to remove the remaining fine particles of dirt.
3. Chlorination
The filtered water is now treated with chlorine to kill the germs. Sometimes, iodine is added to prevent goiter and fluorine to prevent tooth decay. Calcium hydroxide may also be added to remove hardness of water.
The treated water is now clear, free from germs and ready for distribution
Hardness of Water
Water is said to be hard when it does not form lather readily with soap.
Hard water contains a number of dissolved salts including calcium tetraoxosulphate (VI), magnesium tetraoxosulphate (VI) and calcium hydrogen tetraoxosulphate (IV).
When soap – a sodium or potassium salt of an organic acid – is added to hard water, the dissolved salts in the water will immediately react with the soap molecules forming an unpleasant scum which sticks to clothes.
Hard water is wasteful as a large amount of soap is needed to remove the calcium and magnesium ions.
These days, we use detergents for laundry and domestic purposes as they are not affected by hard water
Types of Hard Water
1. Temporary hardness
Water acquires hardness when it dissolves limestone (CaCO3) from the soil or rock over which it flows. Although limestone is insoluble in water, it becomes sparingly soluble when the water contains carbon (IV) oxide
CaCO3 + CO2 + H2O → Ca(HCO3)2
The calcium hydrogen trioxocarbonate (IV) causes temporary hardness and can easily be removed by boiling as it decomposes on heating
Ca(HCO3)2 → CaCO3 + CO2 + H2O
soluble insoluble
Effects of temporary hardness
1. Furring of kettles and boilers
When a kettle has been used to boil temporarily hard water, the inner surface becomes coated with a white fur-like layer due to the gradual deposition of CaCO3
2. Stalactites and stalagmites
These are pillars of limestone found in hot caves. A calcium trioxocarbonate (IV) structure growing downwards from the roof is known as a stalactite while one growing upwards from the floor is known as stalagmite
Temporary hardness can also be removed by using slaked lime
Ca(HCO3)2+Ca(OH)2→2CaCO3+2H2O
Permanent Hardness
This type of hardness can only be removed by using chemicals.
It is caused by the presence of calcium and magnesium ions in the form of soluble tetraoxosulphate (VI) and chlorides
Permanent hardness can be removed by precipitation of the calcium and magnesium ions from solution. The chemicals employed are all soluble sodium compounds which will form insoluble precipitates with the calcium and magnesium ions.
Washing soda (Na2CO3), caustic soda (NaOH) and permutit or zeolite are some of the common chemicals used in the removal of permanent hardness
Na2CO3(aq) + CaSO4(aq) → CaCO3(s) + Na2SO4(aq)
Na2 CO3(aq) + MgSO4(aq) → MgCO3(s) + Na2SO4(aq)
The addition of caustic soda removes the calcium and magnesium ions as the insoluble calcium and magnesium trioxocarbonates (IV) respectively.
2NaOH(aq) + CaSO4(aq) → Ca(OH)2(s) + Na2SO4(aq)
2NaOH(aq) + MgSO4(aq) → Mg(OH)2(s) + Na2SO4(aq)
Permutit or zeolite is an ion-exchange resin used industrially an in the home for softening water. It is naturally occurring sodium aluminium trioxosilicate (IV).
As the hard water is passed through the resin, the sodium ions will go into solution while the unwanted calcium and magnesium ions take their place in the complex salts
Advantages of Hard Water
1.Hard water tastes better than soft water because of the dissolved minerals in it
2.Hard water helps animals such as nails and crabs to make their shells (mainly made of calcium trioxocarbonate (IV)
3.When animals consume hard water, the calcium salts present help them build strong bones and teeth
4.Hard water does not dissolve lead when it is supplied through certain lead pipes but soft water does which can cause lead poisoning
Disadvantages of hard water
1.Hard water causes furring of kettles and boilers while soft water does not
2.Hard water cannot be used in dyeing and tanning as the salts interfere with the modes of action
3.A lot of soap is required before it can form a lather. This is not economical since it leads to unnecessary costs
PRACTICE QUESTIONS
The solubility in mol dm⁻³ of 20g of CuSO₄ dissolved in 100g of water at 180°C is
[Cu = 64, S = 32, O = 16]
When 10g of sodium hydroxide is dissolved in 100cm³ of water, the solution formed is approximately
[Na=23, H=1, O=16]
RATE OF A REACTION
The balanced chemical equation provides no information on the rate of a chemical equation.
Experiments are required to determine the rate at which reactants are used up or the rate products are formed.
The rate of a chemical equation may be defined as follows Rate=(change in reactants or products)/(time taken)
Some chemical processes are fast such as the combustion of petrol, some are slow such as the rusting of iron in the presence of air and moisture.
It is therefore imperative to measure the rate of a chemical equation so as to understand ways to control it.
For example, when you know how long a certain food takes to cook, you know when to stop the cooking process to prevent it from burning.
The branch of Chemistry that deals with reaction rates is called Chemical kinetics.
Chemical kinetics not only helps in finding the rate of a chemical reaction but also the factors influencing it such as temperature, pressure, concentration, catalyst, and so on.
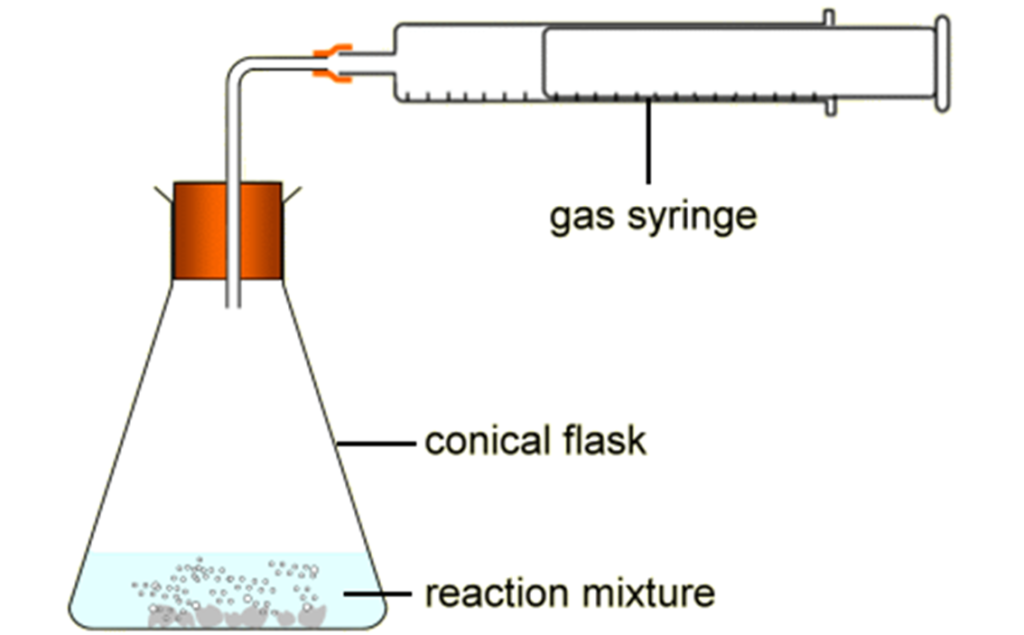
One way to measure the rate of a chemical reaction is to measure the volume of gas for example, if it gives off gas, released in the course of the reaction at regular time intervals
Collision Theory
It simply states that reactant particles (atoms, ions or molecules) must collide with sufficient energy and in the correct orientation before products can be formed.
It is possible for reactant particles to collide and afterwards bounce off from each other without changing. This would be an unsuccessful collision.
Such unsuccessful collisions take place when the colliding particles do not have enough energy to react.
If the reactant particles, however, have enough energy to react, they may change into product particles and we have successful or effective collisions.
According to the collision theory, a reaction will speed up if
•the frequency of collisions increases
•the proportion of particles with energy greater than activation energy increases
Activation Energy
The minimum energy that colliding particles must possess for a successful collision to take place is called the activation energy of that particular reaction.
The activation energy for an exothermic and endothermic reaction can be shown on the enthalpy profile diagrams
*
Factors affecting the rate of a chemical reaction
•Temperature
•Surface area
•Concentration of the solutions
•Pressure of the gases
•Catalyst
•Light
Temperature
As the temperature rises, the average kinetic energy of the molecules increases and they move faster and collide more vigorously thereby causing more frequent collisions.
In a sample of substance at a given temperature, the particles will not all possess the same amount of energy as each other.
A few particles will have a relatively small amount of energy. A few will have relatively large amount of energy.
Most particles will have an amount of energies between.
Increasing the temperature increases the rate of reaction in two ways
1.The increased energy results in particles moving around more rapidly, which in turn increase the frequency of collisions
2.The proportion of effective collisions increases because the proportion of particles exceeding the activation energy increases
Concentration

Recall that we measure concentration in of mol dm^(-3).
The more concentrated a reaction, the greater the number of particles of solute dissolved in a given volume of water.
In reactions involving solutions, more concentrated reactants have a faster rate of reaction.
This is because the random motion of the particles in solution results in more frequent collision between reacting particles
Pressure
The effect of pressure on gases is similar to the effect of concentration of solutions.
As the pressure of reacting gases increases, there are more gas molecules in a given volume. This results in more collisions in a given time, leading to a faster rate of reaction
Surface Area

The more surface area excposed to the attacking reagent, the higher will be the rate of reaction.
Catalyst
A catalyst is a substance that increases the rate of a reaction but remains chemically unchanged itself at the end of the reaction.
A catalyst is able to do this by making it possible for the particles to react by an alternative mechanism.
*
Equilibrium
We can define equilibrium as a state of a system when there is no net observable change in the properties of the system with respect to time.
The concept of equilibrium has to do with reversible reactions.
Many chemical reactions go into completion and cannot be reversed.
For example, when magnesium reacts with excess hydrochloric acid, the reaction stops when all the magnesium has been used up. The products formed cannot be converted into the reactants
Mg + 2HCl → MgCl2 + H2
Some reactions however can be reversed. For example, when blue hydrated copper (II) sulphate is heated, it loses all its water of crystallization and changed to white anhydrous copper (II) sulphate.
CuSO45H2O(s) → CuSO4(s) + 5H2(l)
This is the forward reaction
When water is added to anhydrous copper (II) sulphate, the reaction is reversed
CuSO4(s) + 5H2O(l) → CuSO45H2O(s)
This is the backward reaction
We can show both reactions using a single equation
CuSO45H2O(s) ⇌ CuSO4(s) + 5H2O(l)
Thus a reversible reaction is one in which the products can react to form the original reactants
Another example is ammonia reacting with hydrogen chloride
NH4Cl(s) ⇌ NH3(g) + HCl(g)
Now when the rate at which the forward reaction is proceeding is equal to the rate of backward reaction, the reaction is said to be in equilibrium.
Characteristics of Equilibrium
1. It is dynamic
This means that reactants particles (ions, molecules) are continuously being changed to products and products are continuously being changed to reactants
2. The forward and backward reaction occur at the same rate. The concentrations therefore of reactants and products remain constant at equilibrium
At equilibrium, molecules or ions of reactants becoming products and those in the products becoming reactants, at the same rate.
There is therefore no new change in the concentrations of both reactants and products
3. A chemical equilibrium can be approached from either the point of view of the forward reaction or the backward reaction.
This means that either reactants or products can be used as an initial source for the establishment of equilibrium
In the reaction of H2(g) + I2(g) ⇌ 2Hl(g)
We can start by either
using a mixture of colourless hydrogen gas and purple iodine vapour or
using only colourless hydrogen iodide gas
4. The system is closed and at constant temperature
A closed system is one in which none of the reactants or products are allowed to escape from the reaction mixture.
For example, the equilibrium between hydrogen, iodine and hydrogen iodide can only be achieved in a closed system
H2(g) + I2(g) ⇌ 2HI(g)
In an open system, some matter may be lost to the surroundings
Consider the reaction when calcium trioxocarbonate (IV) is heated in
a closed vessel
an open container
CaCO3(s) ⇌ CaO(s) +CO2(g)
*
Many chemical reactions however can be studied without placing them in closed containers.
They can reach equilibrium in open flask if the reaction takes place entirely in solution and no gas is lost
Factors affecting equilibrium
•Temperature
•Pressure of the reacting system (for gases)
•Concentration of the reacting system
Le Chatellier’s Principle
French chemist, Henri Le Chatellier (1850 – 1936), observed how these factors affect the equilibrium. He put forward a general rule:
If one or more factors that affect an equilibrium is changed (such as temperature, pressure or concentration), the equilibrium will shift so as to annul or neutralize the change.
Position of Equilibrium
Equilibrium position can shift to the left or right.
If a system of equilibrium is disturbed (e.g. by a change in temperature) and more products are obtained or the concentration is increased relative to the reactants, we say that the position of equilibrium has shifted to the right.
If the concentration of reactants is increased relative to the products, we say that that equilibrium has shifted to the left.
We can predict the effect of changing concentration and pressure by referring to the stoichiometry equation for the reaction.
We can predict the effect of changing the temperature by referring to the enthalpy change of the reaction.
Change in concentration on equilibrium
When the concentration of one or more of the reactants is increased:
•the system is no longer in equilibrium
•the position of equilibrium moves to the right to reduce the effect of the increase in concentration of reactants
•more products are then formed until equilibrium is restored
Effects of Pressure on equilibrium
Change in pressure would affect the position of equilibrium when
•One of the reactants or products in the reversible reaction is gaseous.
•The total number of moles of gaseous molecules on the left side of the equation must be different from the total number of moles of gaseous molecules on the right hand side
•The molecules or ions in solids and liquids are packed closely together and cannot be compressed very easily. In gases, the molecules are far apart
*
Pressure
X(g) + Y(g) ⇌ Z(g)
In this reaction, there are two moles of gas on the left and one on the right. It can also be understood, according to Gay-Lussac’s law of combining volumes, as 2 vol of gas on the left and 1 vol on the right.
When the pressure is increased as constant temperature
•The molecules are closer together because the pressure has increased
•The position of equilibrium shifts to minimize the increase
•It shifts in the direction of lower gas moecules
•More product, Z, is formed from X and Y until equilibrium is re-established
Worked Example
2SO2(g) + O2(g) ⇌ 2SO3(g)
There are 3 moles of gas molecules on the reactant side and 2 moles on the product side
What happens when we increase the pressure?
According to Le Chatelier’s principle, the reaction must shift in the direction that reduces the number of molecules of gas
The position of equilibrium shifts to the right
More SO2(g) reacts with O2(g) to form SO3(g)
What happens when we decrease the pressure?
The molecules are further apart, because the pressure is lower
The position of equilibrium shifts to the left
So more SO2(g) and O2(g) molecules are formed by the decomposition of SO3(g) molecules
It is important to note that it is only the molecules of gases that count if the reaction involves gases and solids/liquids
And if there are equal numbers of molecules of gas on each side of the equation , the position of equilibrium is not affected by a change in pressure
Practice Question
Predict the effect of increasing the pressure on the following reactions:
N2O4(g) ⇌ 2NO2(g)
CaCO3(s) ⇌ CaO(s) + CO2(g)
Predict the effect of decreasing the pressure on the reaction
2NO2(g) ⇌ 2NO(g) + O2(g)
Effect of Temperature on equilibrium
Consider the general thermochemical equations,
A+B⇌C ∆H=+ve
X+Y⇌Z ∆H=-ve
•If ∆H is positive, the forward reaction is endothermic and the backward reaction is exothermic
•If ∆H is negative, the forward reaction is exothermic, and the backward reaction is endothermic
In the decomposition of hydrogen iodide for example, the reaction is endothermic
2HI ⇌ H2+ I2 ∆H=+9.6KJ mol-1
The effect of temperature on the equilibrium concentration of hydrogen iodide and hydrogen at equilibrium for the forward reaction is shown in the table
| Temperature /^0 C | Equilibrium conc. Of HI | Equilibrium conc. of H_2 or I_2 |
| 25 | 0.93 | 0.033 |
| 230 | 0.864 | 0.068 |
| 430 | 0.786 | 0.107 |
| 490 | 0.773 | 0.114 |
You notice that as the temperature increases, the concentration of the product increases.
An increase in temperature increases the energy of the surroundings.
According to the Le Chatelier’s principle, the reaction will go to the direction that opposes the increase in energy
The position of equilibrium shifts to the right, producing more H2 and I2
If an endothermic reaction is favoured by an increase in temperature, it follows that an exothermic reaction must be favoured by a decrease in temperature.
2SO2(g) + O2(g) ⇌ 2SO3(g)
An increase in temperature shifts in the position of equilibrium to the left and more reactants (SO2 and O2) are formed.
A decrease in temperature shifts the equilibrium position towards the product
Practice Question
1. Predict the effect of increasing the temperature on the reaction:
H2(g) + CO2(g) ⇌ H2O(g) + CO(g)
∆H=+41.2KJ mol^(-1)
2. In the reaction
Ag2CO3(s) ⇌Ag2O(s) + CO2(g)
Increasing the temperature increases the amount of carbon dioxide formed at constant pressure. Is this reaction exothermic or endothermic?
Effect of a catalyst on equilibrium
Recall that a catalyst is a substance that increases the rate of a chemical reaction.
Catalysts speed up the time taken to reach equilibrium, but have no effect on the equilibrium position once this is reached.
This is because they increase both the forward and backward reactions equally
Equilibrium Constant
For a general reaction:
mA + nB ⇌ pC+ qD
Where m, n, p and q are the number of moles in the equation
K_c=([C]^p [D]^q)/([A]^m [B]^n )
Equilibrium Expression
When hydrogen reacts with iodine in a closed tube at 500K, the following equilibrium is set up:
H2 + I2 ⇌ 2HI
The equilibrium expression is given as
K_c=[HI]^2/[H_2 ][I_2 ]+
The square brackets refer to the concentration in mol dm^(-3) of the substance inside the brackets
In equilibrium expressions involving a solid, we ignore the solid
Ag+(aq)+ Fe2+(aq)) ⇌ Ag(s) + Fe3+(aq)
What is the unit of Kc?
Kc= Fe(3+)(aq)/ Ag+(aq) Fe(2+)(aq)
It depends on the form of the equilibrium expression
Worked Examples
1. Write the equilibrium expressions for the following reactions and state the unit of K_c
a) CO(g) + 2H2(g) ⇌ CH3 OH(g)
b) 4HCl(g) + O2(g) ⇌ 2H2 O(g) + 2Cl2(g)
c) N2(g) + 3H2(g) ⇌ 2NH3(g)
Change in Concentration and Equilibrium Constant
What happens to the equilibrium constant when the concentration of products (or reactants) are altered?
Consider the decomposition of hydrogen iodide
2HI ⇌ H2 + I2
The equilibrium constant at 500K for this reaction is 6.25 × 10(-3) Kc = H2 I2 /HI2
When more hydrogen iodide is added to the equilibrium mixture, the equilibrium is disturbed
The ratio of concentration of products to reactants in the equilibrium expression decreases
To restore equilibrium, the concentration of H2 and I2 increases while [HI] decreases
Equilibrium is restored when the values of the concentration in the equilibrium expression are such that the value of Kc is once again 6.25×10(-3)
Hence, the value of Kc does not change when the concentration of reactants or products is altered as long as other conditions are kept constant
Change in Pressure and Kc
We have already understood that when there are different numbers of gas molecules on each side of a chemical equation, a change in pressure alters the position of equilibrium.
It is shifted in the direction that results in fewer gas molecules being formed
However, if all other conditions remain constant, the value of Kc does not change when the pressure is altered
Changes in temperature and Kc
We have seen that for an endothermic reaction, an increase in temperature shifts the reaction in the direction of more products.
So for the reaction N2 + O2⇌ 2NO ∆H=+90.4KJ mol(-1)
The forward reaction is endothermic
Increasing the temperature of the system will shift the equilibrium position to the right, favouring the forward reaction, i.e. product formation.
Hence, the value of equilibrium constant K also increase. So, for a given reversible reaction, a higher value of K would mean a greater yield of product(s).
Lowering the temperature of the system will shift the equilibrium position to the left. This results in a lowering of the K value. So, for a given reversible reaction, a lower value of K would mean a greater yield of reactant(s)
In the following reaction, the forward reaction is exothermic
2SO2(g) + O2(g) ⇌ 2SO3(g) ∆H=-395.7KJ mol(-1)
An increase in temperature will cause the equilibrium position to shift to the let i.e. it favours reactant formation. Equilibrium constant will decrease
Practice Question
Deduce the effect of increase and temperature on the value of Kc for the reaction:
2NO2(g) + O2(g) ⇌ 2NO(g) ∆H_f=-115KJ mol(-1)
Equilibria and the Chemical Industry
In industrial processes involving reversible reactions, the concept of chemical equilibrium and Le Chatelier’s principle are applied to determine the optimum conditions of operation. Industrial chemists try to
•Minimize cost of production by ensuring that the starting materials are cheap and the capital cost of the plant is not too high
•Maximize yield of products by shifting equilibrium to the desired position in the desired direction and increasing the value of equilibrium constant
•Ensure that the shortest possible time is taken to reach equilibrium.
We shall now look at some important processes
Haber’s process
The industrial production of ammonia is carried out by the Haber’s process.
The equilibrium reaction is
N2(g) + 3H2(g) ⇌ 2NH3(g) ∆H_r=-92kJ mol(-1)
We can use Le Chatelier’s principle to show how to get the best yield of ammonia.
The forward reaction is exothermic, decreasing the temperature will give a high yield of ammonia. The position of equilibrium will shift to the right and the value of Kp increases.
Thus, the temperature of the system is kept as low as 4500C. Lower temperatures of 2000C and 3000C give better yields of ammonia, but are not economically feasible as it will take too long for the reaction system to attain equilibrium. This is because the rate of reaction decreases as temperature decreases
What happens if we increase the pressure?
Then according to Le Chatelier’s principle, the equilibrium position will shift in the direction of lesser molecules, that is, the forward reaction and the yield of ammonia increases.
The Haber’s process is always operated at very high pressures of about 200 atm in order to get high yield of ammonia.
A higher pressure, such as 1000 atm, would give a higher yield. However, it would be costly to build production plants that would be strong enough to withstand such a high pressure.
To further increase the yield of ammonia, the raw materials (hydrogen and nitrogen) are supplied continuously while the ammonia produced is removed continuously by liquefaction before returning to nitrogen and hydrogen.
In addition, the production time of the process is shortened by using small pellets o iron to act as a catalyst. The catalyst speeds up the rate of reaction enabling equilibrium to e attained in a shorter time
The Contact Process
In the production of tetraoxosulphate (VI) acid, the contact process comes to mind.
The main equilibrium reaction involved is
2SO2(g) + O2(g) ⇌ 2SO3(g) ∆H_r=-197KJ mol(-1)
When the pressure is increased, the reaction goes in the direction that results in fewer molecules of gas being formed. In this case, equilibrium shifts to the right since there are 3 moles of gas molecules on the reactant side and 2 moles on the product side.
In practice however, the reaction is carried out at just atmospheric pressure to give a high yield of sulphur (VI) oxide (98%). This is because the value of kp is very high. The equilibrium is far over to the right even at atmospheric pressure. Very high pressure is unnecessary, and is not used as it is expensive.
What happens when we decrease temperature?
Since the reaction is exothermic, a decrease in temperature decreases the energy of the surroundings so the reaction will go in the direction in energy is released. Thus, the position of equilibrium sill shift to the right.
Practically, a temperature of 450 -5000C and a catalyst (Vanadium (V) oxide) is used. These conditions increase the reaction rates and enable equilibrium to be reached in a shorter time, thus making the process economical feasible.
SO3 is removed y absorbing it in 98% tetraoxosulphate (VI) acid.
Although the SO3 is absorbed continously, this does not affect the equilibrium significantly because the position of equilibrium is already far over to the right
PRACTICE QUESTIONS
ENTHALPY
Enthalpy is the total energy associated with the materials that react.
We cannot measure enthalpy, but we can measure an enthalpy change when heat energy is exchanged with the surroundings
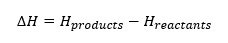
The units of enthalpy change are kilojoules per mole (KJ mol-1)
We can draw enthalpy profile diagrams or reaction pathway diagrams to show enthalpy changes.
The enthalpy of the reactants and products is shown on the y-axis. The x-axis shows the reaction pathway, with reactants on the left and products on the right
We know that in an exothermic reaction, energy is released to the surroundings. So the enthalpy of the reactants must be greater than the enthalpy of the products.
We can see from the enthalpy profile diagram for the combustion of methane that H_products-H_reactants is negative.
CH4(g)+2O2(g) →CO2(g)+2H2 O(l)
∆H=-890.3 KJ mol-1
The negative sign shoes that the reaction is exothermic

To make fair comparison of enthalpy changes, we must use the same conditions, which we refer to as standard conditions.
A pressure of 1.01×105 Pa, approximately normal atmospheric pressure
A temperature of 298k (250 C)
Each substance involved in the reaction in its normal physical state
We can describe enthalpy changes according to the type of reaction
Enthalpy change of formation
Enthalpy change of combustion
Enthalpy change of neutralization
Enthalpy change of solution
Enthalpy change of atomization
Enthalpy change of hydration
The standard enthalpy change of formation ∆H_fis the heat absorbed or evolved when 1 mole of a compound is formed from its elements under standard conditions
Fe(s)+O2(s)→Fe2 O3(s)
2Fe(s)+1 1/2 O2(s)→Fe2 O3(s)
∆Hf [Fe2 O3(s)]=-824.2KJ mol-1
By definition, the standard enthalpy change of formation for an element in its standard state is zero
GASES AND GAS LAW
We have already explained the characteristics of gases from the perspective of the molecules that make them up. We said concerning them
The particles have much more kinetic energy than those of a solid or liquid.
The cohesive forces between gas particles are negligible and they are free to move about in all directions.
A gas has no definite shape or size and they occupy the whole volume of the container
They can be easily compressed
A change of state (between solid, liquid and gas) is brought about by a temperature change, that is, heating or cooling.
Assumptions of the Kinetic Theory of Gases
The gas molecules move randomly in straight lines
The collisions of the gas molecules are perfectly elastic
The actual volume of the gas molecules themselves is negligible compared with the volume of their containing vessel
The cohesive forces between the gas molecules are negligible
The temperature of the gas is a measure of the average kinetic energy
Gas Laws
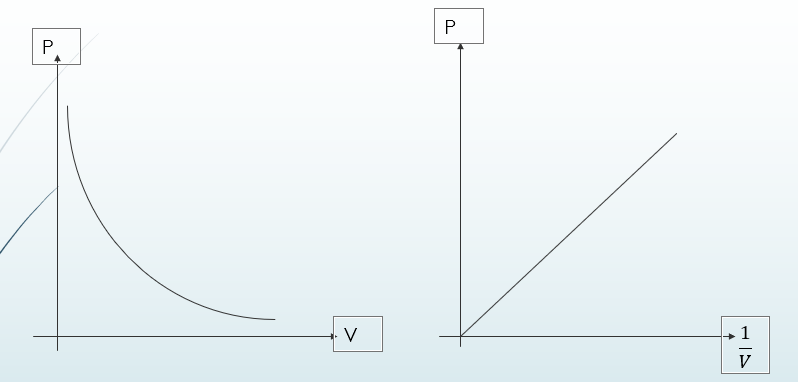
Boyle’s Law
It states that the pressure of a fixed mass of gas is inversely proportional to the volume provided temperature is kept constant.
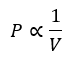
PV=constant
Example
360cm3 of a gas has pressure of 770mm Hg. Find its volume if the pressure is reduced to 750mm Hg.
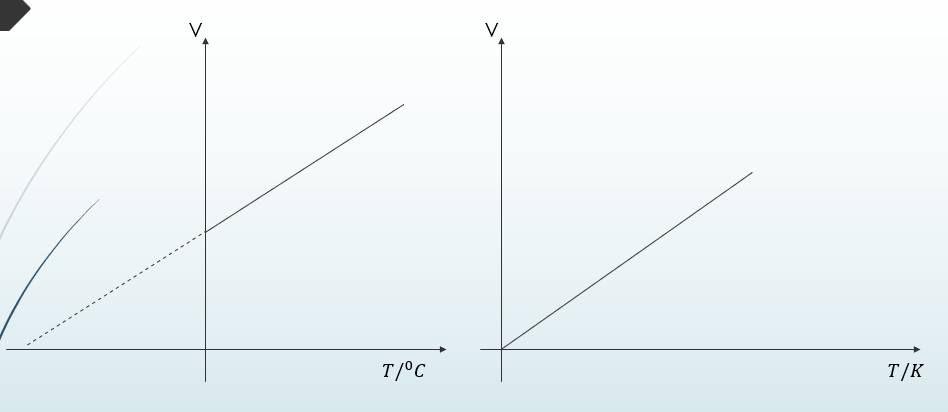
Charles Law
It states that the volume of a fixed mass of gas is directly proportional to the absolute temperature provided pressure is kept constant
V∝T
V/T=constant
P=pressure, V=volume, and k is a constant of proportionality
Boyle’s law explains that for any given mass of gas, the product of its pressure and its volume is always constant. If the pressure increases, the volume will decrease by a similar proportion.
This relationship can also be expressed in the form P1 V1=P2 V2
Pressure Law
It states that the pressure of a gas is directly proportional to the absolute temperature
P∝T
General Gas equation
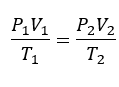
Ideal Gas Equation
PV=nRT
R=molar gas constant=8.314 J mol-1 K-1
Dalton’s law of Partial Pressure
If a mixture contains gases which do not react chemically together, then the total pressure is equal to the sum of partial pressures of the individual gases.
If PA, PB, PC are the partial pressures of gases A, B and C that make up the mixture, then
Ptotal=PA+PB+PC
If a gas is collected over water, it is likely to be saturated with water vapour, and the total pressure becomes
Ptotal=Pgas+Pwater vapour
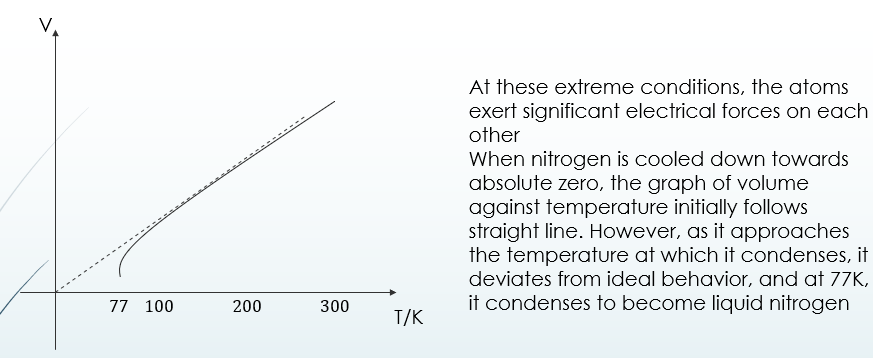
Real and Ideal Gases
Ideal gases are those gases that obey the gas laws exactly embodied in the ideal gas equation PV=nRT
Unfortunately, no such gas exists.
At low pressures and temperatures above which they liquefy, they provide a fairly accurate description of ideal
However, when extreme conditions such as low temperatures or high pressures are employed, gases start to deviate from these laws
Gay Lussac’s Law
It states that when gases react they do so in volumes which are simple ratios to one another and the volume of product if gaseous, provided temperature and pressure are kept constant.
Example
What is the volume of oxygen required to burn completely 45cm3 of methane
CH4+2O2→CO2+2H2 O
Avogadro’s Law
Equal volumes of all gases at the same temperature and pressure contain the same number of molecules
This law led to the Avogadro’s number , 6.02×1023
Thus, 1 mole of diatomic Nitrogen or carbon (IV) oxide contains 6.02×1023 molecules
Molar Volume
1 mole of a gas at standard temperature and pressure occupies a volume of 22.4dm3.
Graham’s law of diffusion
The rate of diffusion of a gas is inversely proportional to the square root of its relative molecular mass or vapour density.
R∝1/√M
However, when extreme conditions such as low temperatures or high pressures are employed, gases start to deviate from these laws
Practice Questions
Consider the following reaction equation: 2H2(g) + 02(g) → 2H20 (g) Calculate the volume of unused oxygen gas when 40cm3 of hydrogen gas is sparked with 30cm3 of oxygen gas.
Calcium carbonate of mass 1.0 g was heated until there was no further change. Write an equation for the reaction which took place i. Calculate the mass of the residue ii. Calculate the volume of the gresidue
ii. Calculate the volume of the gas evolved at s.t.p
ii. What would be the volume of the gas measured at 15°C and 760mmHg?
[C = 12.0, O = 16.0, Ca = 40.0, molar volume of a gas at s.t.p. = 22.4dm3
When a given mass of CaCO3 was heated, 0.25dm3 of a gas is collected at 250°C and at a pressure of 120 kNm-2 (i) Write an equation for the reaction (ii)
Calculate the mass of the:
I. gaseous product obtained
II. CaCO3 heated
[C = 12.0, 0 = 16.0, Ca = 40.0, R = 8.314JK-1mol-1]
ACIDS, BASES & SALTS
ACIDS
The sour taste of some fruits such as lemon or lime have been known to be acids.
There are two classes of acids – inorganic or organic acids
Organic acids can be found in plants or animals while inorganic can be prepared from elements or inorganic matter
| Inorganic acid | Formula |
| Hydrochloric acid | HCl |
| Nitric acid (trioxonitrate (V) acid) | HNO3 |
| Sulphuric acid (tetraoxosulphate (VI) acid) | H2SO4 |
| Organic acid | Source |
| Citric acid | Lime, lemon |
| Lactic acid | Milk |
| Ascorbic acid | Oranges |
| Ethanoic acid | Vinegar |
| Amino acids | Proteins |
| Fatty acids | Fats and oils |
We shall be studying only inorganic acids.
Do you notice that each of these acids have one common element in them?
An acid is a substance which produces hydrogen ion as the only positive ion when dissolved in water.
So we conclude that a substance is not an acid if it doesn’t produce hydrogen ions.
Or we can put it in another way by saying that an acid must have at least one ionizable hydrogen atom in its molecule
This is correct because dry hydrogen chloride gas HCl(g) does not form hydrogen ion when dissolved in methylbenzene and thus doesn’t show acidic properties
But when it is dissolved in water, it produces hydrogen ion and then behaves like a typical acid HCl(aq)
Strong acids ionize completely in water to give hydrogen ions and other negative ions (anions). The concentration of hydrogen ions is very high in such acid solutions.
H2SO4, HCl and HNO3 are strong acids
HCl(aq) → H+ + Cl–
HNO3(aq) → H+ + NO3–
H2SO4(aq) → 2H+ + SO42-
Weak acids are only partially ionized in water and have a low concentration of hydrogen ions. For example, ethanoic acid CH3COOH(aq) has only 0.4% ionization in water. This means that only 4 out of every 1000 acid molecules ionize in water.
You may wish to note that scientists come about this conclusion of strong and weak acids from experimental analysis and not by merely looking at the molecular formula or equation.
Other examples of weak acids include trioxocarbonate (IV) acid (H2CO3), tetraoxosulphate (IV) acid (H2SO3), tetraoxophosphate (V) acid (H3PO4) and most organic acids
CH3COOH(aq) ⇌ H+ + CH3COO–
H2CO3(aq) ⇌ 2H+ + CO32-
H2PO4(aq) ⇌ 3H+ + PO43-
H2SO3(aq) ⇌ 2H+ + SO32-
Note also that there is a difference between a weak acid and a dilute acid (or a strong acid and a concentrated acid).
If a large amount of water is added to a small amount of acid, the resulting solution is dilute. If however, a little amount of water is added to large amount of acid, then the resulting solution will be concentrated.
Thus conc. H2SO3(aq) will burn the skin if not handled properly
Basicity of an acid
This is the number of replaceable hydrogen ion in one molecule of the acid
| Acid | Ions Produced | Basicity |
| HCl | H+, Cl– | 1 (monobasic) |
| CH3COOH | H+, CH3COO– | 1 (monobasic) |
| H2SO4 | 2H+, SO42- | 2 (dibasic |
| H2PO4 | 3H+, PO43- | 3(tribasic) |
Did you notice that although ethanoic acid has 4 atoms of hydrogen, only 1 is replaceable?
Physical properties of acids
- Concentrated forms of acids are corrosive (they can burn) while dilute acids have a sour taste.
- Acids turn blue litmus paper red
PRECAUTION: Do not add water directly to acid (just as you do not add water directly to hot cooking oil). Always add acid to water
Chemical Properties of acids
- Acids react with metals to liberate hydrogen gas
acid + metal → salt + hydrogen
2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
H2SO4(aq) + Na(s) → Na2SO4(aq) + H2(g)
Trioxonitrate (V) acid is an exception to this rule
Also less electropositive metals like gold (Au), silver (Ag) do not react with acids. This is so because they are not ‘strong’ enough to displace the hydrogen ions from the acid
2. Acids react with bases to produce salt and water only. This is commonly referred to as a neutralization reaction.
acid + base → salt + water
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
H2SO4 + Ca(OH)2→ CaSO4+ 2H2O
3. Acids react with trioxocarbonates to liberate carbon (IV) oxide
acid + trioxocarbonate (IV) → salt + water + carbon(IV)oxide
HCl(aq) + CaCO3(s) → CaCl2(aq) + H2O(l) + CO2(g)
Preparation of acids
1. Dissolving an acid anhydride or acidic oxide in water
CO2(g) + H2O(l) → H2CO3(aq)
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
2. Combination of the acid’s elements
H2(g) + Cl2(g) → 2HCl(g) (with activated charcoal as catallyst)
Hydrogen chloride can now be dissolved in water to produce hydrochloric acid
3. Displacement reaction
A strong acid may be used to displace a weaker acid or a more volatile acid from its salt
H2SO4(aq) + 2NaNO3(aq) → Na2SO4(aq) +2HNO3(aq)
H2SO4(aq) + 2KCl(aq) → K2SO4(aq) +2HCl(g)
Test for Acids
An acid turns blue litmus paper red
Uses of Acids
- Hydrochloric acid is used to remove rust and also to produce to other chemicals
- Trioxonitrate (V) acid is used to make fertilizers and explosives
- Tetraoxosulphate (VI) acid is used as a drying and dehydrating agent. It is also used as an electrolyte in car-batteries (lead-acid accumulators). It is employed in industries to make chemicals and also required in oil refineries
- Citric acid is used in making fruit juice
- Acetic acid (ethanoic acid) is used in the preservation of food as well as dyeing of silk and other textiles
- Fatty acid are used in the production of soap
- Tartaric acid is used in making soft drinks and baking soda
- Boric acid is used as a mild antiseptic or germicide
BASES and ALKALIS
When metals burn in air or oxygen, a metallic oxide is formed.
4Na(s) +O2(g) → 2Na2O(s)
Most oxides and hydroxides of metals are bases. Common examples of basic oxides include sodium oxide, Na2O, potassium oxide, K2O, magnesium oxide, MgO
Most of these oxides are insoluble in water. The ones that dissolve form hydroxides
Na2O(s) + H2O(l) → 2NaOH(aq)
A soluble hydroxide such as NaOH, KOH, Ca(OH)2 is known as an alkali.
An alkali is a soluble hydroxide
Does copper (II) oxide, zinc oxide or aluminum oxide dissolve in water?
Like acids, alkalis may be strong or weak depending on how they ionize in water.
NaOH(aq) → Na+ + OH–
KOH(aq) → K+ + OH–
Sodium hydroxide and potassium hydroxide are strong alkalis because they ionize completely in aqueous solutions to produce negatively charged hydroxide ions OH–
Calcium hydroxide and aqueous ammonia are weak alkalis because they ionize only partially.
Ca(OH)2 ⇌ Ca2+ + 2OH–
NH4OH ⇌ NH4+ + OH–
Physical properties of alkalis
- Concentrated forms of NaOH and KOH are corrosive
- Alkalis have bitter taste
- They have a soapy feel
- Alkalis turn red litmus paper blue
Chemical properties of alkalis
- Neutralization reaction
Bases or alkalis react with acids to form salt and water only
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
MgO(s) + 2HNO3(aq) → Mg(NO3)2(aq) +H2O(l)
2. Alkalis react with ammonium salts to liberate ammonia
NaOH(aq) + NH4NO3(aq) → NaNO3(aq) + H2O(l) + NH3(g)
Uses of Alkalis
- Sodium hydroxide is used in the manufacture of soap and plastics. It is also used in petrol refining
- Potassium hydroxide is used in dyeing and electroplating as well as manufacture of liquid soap
- Magnesium hydroxide is used to produce toothpaste and also used as a laxative
- Calcium hydroxide is used in the manufacture of mortar, plaster and cement
- Aqueous ammonia is used for bleaching cloth
SALTS
To a layman, a salt refers to the common table salt (sodium chloride) used for seasoning food. To a chemist however, a salt means a compound found when the hydrogen ion is replaced in an acid.
A salt is formed when all or part of the ionizable hydrogen ion from an acid is replaced by a metal ion or ammonium ion.
If the ions of the metals sodium, potassium or calcium replace hydrogen ion in HCl, we have the salts
NaCl, KCl, CaCl2
in H2SO4, we have the salts Na2SO4, k2SO4, or CaSO4–
Types of Salts
1. Normal salt
Normal salts are formed when all of the replaceable hydrogen ions have been replaced by metallic ions
HCl(aq) + NaOH(aq) → NaCl(aq)+ H2O(l)
H2SO4(aq) + ZnO(s) → ZnSO4(aq) +H2O(l)
Normal salts are usually neutral to litmus. There are few normal salts however that give an acidic or alkaline medium when they undergo hydrolysis in water. Some of them include sodium trioxocarbonate (IV), aluminium chloride and sodium sulphide
2. Acid salts
They are formed when the replaceable hydrogen ions in acids are only partially replaced by a metal.
H2SO4(aq) + KOH(aq) → KHSO4(aq) +H2O(l)
3. Basic salts
Basic salts contain the hydroxide ion, OH–
These salts occur when there is an insufficient supply of acid which is needed for the complete neutralization of the base.
Ca(OH)2(aq) + HCl(as) → Ca(OH)Cl(aq) + H2O(l)
Basic salts still have the properties of a base
4. Double salts
These salts ionize to produce different types of ions in solution. Usually, two of these ions will be positively while the other will be negatively charged.
Example of double salt include potash alum, KAl(SO4)2.12H2O
(NH4)2Fe(SO4)2.6H2O
5. Complex Salts
They are formed by mixing together two simple salts. Example is the salt formed from tetraammine copper (II) ion [Cu(NH3)4]2+
Uses of Salts
Salts are used as preservatives, drying agents and antifreeze
They are also used to manufacture many industrial, agricultural and consumer substances such as fertilizers, chlorine gas, laxatives
Preparation of Salts
| Soluble Salts | Insoluble Salts |
| All sodium, potassium and ammonium salts | |
| All trioxonitrates | |
| All chlorides except | Lead (II) chloride and silver chloride |
| All tetraoxosuphates (VI) except | Lead (II) – & Barium tetraoxosulphate (VI) |
| The trioxocarbonates (IV) of sodium, potassium and ammonium | Other trioxocarbonates (IV) |
Preparation of Soluble Salts
1. Dilute acid and metal
2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
2. Alkali and acid
HCl(aq) + NaOH(aq) → NaCl(aq)+ H2O(l)
3. Dilute acid and insoluble base
H2SO4(aq) + CuO(s) → CuSO4(s) +H2O(l)
4. Dilute acid and trioxocarbonate (IV)
HCl(aq) + CaCO3(s) → CaCl2(aq) + H2O(l) + CO2(g)
Preparation of Insoluble Salt
- Double Decomposition
This involves the exchange of radicals of two soluble compounds. One containing the metallic radical and the other containing the acidic radical of the required insoluble salt
AgNO3 + NaCl→ AgCl + NaNO3
BaCl + K2SO4 → BaSO4 + KCl
2. Combination of Constituents elements
Salts such as chlorides or sulphides of metals can be prepared using this method.
Fe + S → FeS
2Fe + 3Cl2 → 3FeCl3
Water of Crystallization
Many salts combine chemically with water to form hydrates
e.g CuSO4 + 5H2O → CuSO4.5H2O
NON-METALS and COMPOUNDS
HYDROGEN
Cavendish is regarded as the discoverer of hydrogen because he was the first (in 1766) to prepare it in the pure state, describe its properties and recognize it as an element. He found that the gas was flammable and that it burned in air to produce water. Therefore. Lavoisier named it hydrogen, meaning water-former.
Occurrence:
Hydrogen makes up approximately 1% of the earth’s crust. It is found free only in very small amounts (0.01%) in the atmosphere and in volcanic gases, although recent spectroscopic studies show that large amounts are present in the sun and the stars.
Hydrogen is widely distributed in combination. with other elements. It makes up one ninth by mass of water and is an important constituent of all acids and alkalis. Combined with carbon, hydrogen is found in natural gas, kerosene, gasoline and other petroleum products. It is also a constituent of most other organic substances, including proteins, carbohydrates and fats which are essential components of all living matter.
Electronic Configuration
H₂ = Is¹
Owing to its configuration, hydrogen has only one electron in its shell. It, therefore, needs one other electron to complete its octet (Is²) and so resemble helium (Is²).
Oxidation Number of H₂:
It has an oxidation number of 1 in most of its compounds except hydrides where it is –1
Laboratory Preparation:
Hydrogen is liberated when certain metals react with with dilute mineral acids, water or steam. It is also given off when tin, zinc or aluminum reacts with hot concentrated solutions of sodium or potassium hydroxide. The three methods commonly used for the preparation of hydrogen in the laboratory are as follows:
Action of zinc on an acid: Dilute hydrochloric or tetraoxosulphate(VI) acid attacks metallic zine with the liberation of hydrogen gas. No heating is necessary.
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Zn(s) + H₂SO₄(aq) → ZnSO₄(aq) + H₂(g)
Ionically, both these reactions can be represented as follows:
Zn(s) + 2H⁺(aq) → Zn²⁺(aq) + H₂(g)
Action of sodium on cold water: Sodium liberates hydrogen from cold water. This reaction is very vigorous and should be carried out with extreme care using only a small piece of sodium.
2Na(s) + 2H₂O(l)→ 2NaOH(aq) + H₂(g)
Action of iron on steam: Iron, at red heat liberates hydrogen from steam. Iron(II) diiron(III); oxide.Fe,O,, is formed at the same time. This reaction is reversible.
3Fe(s) + 4H₂O(g) ⇋ Fe₃O₄(s) + 4H₂(g)
Industrial Preparation
From water gas (Bosch process): In this process large quantities of hydrogen are produced from cheap raw materials, namely water and coke. When steam is passed over red-hot coke (carbon) at about 1 200°C, a mixture of carbon(II) oxide and hydrogen known as water gas is produced. Excess steam is then mixed with the water gas and passed over a catalyst, iron(III) oxide or chromium(III) oxide, at 450°C. As a result, the carbon(II) oxide in the water gas is converted to carbon(IV) oxide with a further yield of hydrogen. The first reaction, i.e:
-the production of water gas, is endothermic, while the second, i.e.
-the reduction of steam to hydrogen by carbon(II) oxide, is exothermic.
Endothermic Reaction H₂O(g) + C(s)→ CO(g) + H₂(g)
Exothermic reaction CO(g) + H₂(g) + H₂O(g) → CO₂(g) + 2H₂(g)
The carbon(IV) oxide is then removed from the mixture by dissolving it in water (under a pressure of 30 atmospheres) or other solvents such as caustic soda solution. Any unreacted carbon(II) oxide is absorbed in an ammoniacal solution of copper(1) ethanoate.
From methane: The Bosch process is now being replaced by a similar process which uses cheap hydrocarbons like methane instead of coke. For example, in the first stage, steam is mixed with methane (obtained as a natural gas) and passed over a nickel catalyst at about 800°C. The mixture of carbon(II) oxide and hydrogen produced is known as synthesis gas.
CH,(g) + H,O(g) → CO(g) + 3H (g)
The second stage in this process is exactly the same as the water gas-steam reaction in the Bosch process.
By electrolytic methods: Very pure hydrogen is obtained as a by-product in the electrolysis of brine for the manufacture of sodium hydroxide and chlorine. If required, it can also be made by the electrolysis of dilute sodium or potassium hydroxide solution.
Hydrogen and the Activity Series
In the laboratory preparation of hydrogen, we saw that some metals could displace hydrogen from water and acids. There are, however, other metals like copper and silver which cannot displace hydrogen from water or acids. We can regard metals which displace hydrogen from water and acids as being more active than hydrogen, and those that do not as being less active.
The metals which can displace hydrogen from water or acids vary in their degree of activity too Potassium and sodium react vigorously with cold water to displace hydrogen, while calcium reacts slowly. Heated magnesium displaces hydrogen from steam, aluminium, zinc and iron will only do so at red heat; while lead and copper do not react at all.
Decreasing activity with water: K, Na, Ca, Mg, Al, Zn. Fe, Sn, Pb, Cu, Hg. Ag. Au
With dilute hydrochloric or tetraoxosulphate(VI) acid, potassium and sodium react too violently for the reaction to be carried out safely in the laboratory Calcium and magnesium react vigorously, while aluminium, zinc and iron react moderately, with the rate of reaction (as measured by the rate of release of hydrogen bubbles) being the fastest with aluminium and the slowest with iron. Lead and copper do not react with dilute acids.
NOTE: The pieces of metals should be cleaned to remove oxides before being placed in the dilute acid solution. Lead does not show any reaction with the dilute acids because the lead salts formed are insoluble and form a protective coating over the metal.
We can also determine the relative activity of metals by doing another set of experiments where one metal displaces another from a solution of its salt. For example, if an iron rod is dipped in a solution of a copper(II) salt, reddish-brown deposits of metallic copper are found on the iron rod after some time, showing that iron has displaced copper from a solution of a copper(II) salt. No displacement occurs if a copper rod is dipped in a solution of an iron salt. However, metallic copper can displace silver from a solution of a silver salt. Thus, iron is more reactive than copper, while the latter is more reactive than silver.
From the above experiments, we can arrange the various metals in an activity series as shown. This series is very similar to the electrochemical series. Hydrogen is placed in the activity series to indicate the position it would occupy. Metals above hydrogen in the series liberate hydrogen from acids, while those below hydrogen do not. Generally, metals high up in the series are very electropositive. They are very active chemically. Metals low down in the series are less active. Any metal will displace those metals below it from a solution of their salts. Thus, the activity series is a useful guide to the chemical behaviour of metals and their compounds.
Physical Properties
1.Hydrogen is a colourless, odourless and tasteless gas.
2.It is neutral to moist litmus paper.
3.It is relatively insoluble in water. Only 2cm³ of it will dissolve in 100cm³ of water at s.t.p.
4.Hydrogen is the lightest known substance. It is 14.4 times less dense than air.
5.It has a very low boiling point of -253°C.
Chemical Properties
Hydrogen is an unusual element. It has a single valence electron like the Group I alkali metals, yet it is clearly a gas with non-metallic properties like the Group 7 halogens. In the Periodic Table, hydrogen is usually placed in Group 1 for convenience. However, sometimes it is placed between Groups 1 and 7 to depict its unusual nature
The chemical behaviour of hydrogen can be explained by its tendency to acquire the stable duplet electronic configuration of helium. Thus, hydrogen:
-accepts an electron from another atom to form the negative hydride ion, H⁻
-forms a covalent bond by sharing its lone electron as in the hydrogen molecule, H—H;
-donates its lone electron to form the positive hydrogen ion, H⁺ and enters into coordinate bond formation with molecules having lone pairs of electrons. For example, hydrogen ion combines with water to form the hydroxonium ion. H₃O⁻ and with ammonia to form the ammonium ion, NH₄⁺
Combination Reactions
Hydrogen combines with certain metals and non metals to form electrovalent and covalent hydrides respectively. In both instances, the hydrogen atom gains an extra electron to achieve the stable electronic configuration of helium.
With metals: Hydrogen combines directly with several of the more active metals to form ionic hydrides, ie compounds which contain the hydride ion, H⁻. For example,
2Na(s) + H₂(g) → 2NaH(s)
With oxygen: Pure hydrogen burns with a pale blue flame as it combines with oxygen to produce steam.
2H₂(g) + O₂(g) → 2H₂O(g)
With halogens: Hydrogen combines directly with the halogens to produce halides. For example,
Cl₂(g) + H₂(g) → 2HCl(g)
Br₂(g) + H₂(g) 2HBr(g)
The reaction between hydrogen and chlorine is spontaneous in bright sunlight but slower in diffused light. The combination of hydrogen with bromine and with iodine is much less vigorous.
Reducing action
Hydrogen is a strong reducing agent. It reduces the oxides of copper, lead, iron and zinc to the respective metals when they are heated in a stream of the gas. At the same time, the hydrogen itself is oxidized to form water.
CuO(s) + H₂(g) → Cu(s) + H₂O(g)
Fe₂O₃(s) + 3H₂(g) → 2Fe(s) + 3H₂O(g)
Test for hydrogen
Insert a lighted splinter into a test-tube containing the unknown gas. If the gas is hydrogen, it will burn with a pop sound, since it will invariably mix with the air as soon as the test-tube is unstopped. This test should only be carried out with small quantities of the gas.
Uses
1.Hydrogen is used in the manufacture of ammonia, hydrochloric acid and methanol.
2.Hydrogen under high pressure is passed through vegetable oils (e.g. palm oil, corn oil, cotton seed oil or soybean oil) in the presence of a nickel catalyst to give solid fats, which are used as margarine or as lard substitutes, and in the manufacture of soap and candles.
3.Since hydrogen has a low density, it is used for filling balloons. Its highly flammable nature, however, limits this use to meteorological studies and other scientific purposes.
4.Hydrogen is passed through a mixture of oil and powdered coal at high temperatures and pressures to yield a mixture of hydrocarbons from which synthetic petrol can be extracted by fractional distillation. This petrol is more expensive than ordinary petrol and is used in countries with plenty of coal but no petrol.
5.Hydrogen is a constituent of many gaseous fuels such as water gas and coal gas, Liquid hydrogen is also used as a rocket fuel.
6.Hydrogen gives out a lot of heat when it burns. Thus it is used in oxy-hydrogen flames to produce high temperatures (over 2 000°C) that can melt metals. Hydrogen is also used in atomic hydrogen flames. When hydrogen is passed through an electric arc, its molecules absorb energy and break up to form atoms. These atoms recombine when they are out of the arc, evolving large amounts of energy in the form of heat.
Isotopes of Hydrogen
Hydrogen exists in three isotopic forms, namely hydrogen or protium, ¹₁H, heavy hydrogen or deuterium, ²₁H or D, and tritium, ³₁H or T, with relative atomic masses of 1, 2 and 3 respectively. Deuterium is chemically similar to protium except that it is slightly less reactive. Deuterium also forms an oxide. D₂O. which is similar to water, H₂O. Deuterium oxide is commonly known as heavy water because it is about 1.1 times heavier than water. Tritium is radioactive and is rarely found in ordinary hydrogen.
HYDRIDES
The alkali and alkali-earth metals like sodium and calcium form ionic hydrides with hydrogen. These hydrides are crystalline solids with high melting points that conduct electricity when molten. They readily react with water to form hydroxides and liberate hydrogen gas. For example.
CaH₂(s) + 2H₂O(l) → Ca(OH)₂(aq) + 2H₂(g)
Ionically,
H⁻(s) + H₂O(l) → OH⁻(aq) + H₂(g)
Boron and aluminium form complex covalent hydrides which are important reducing agents especially in organic chemistry. The two common complex hydrides are lithium tetrahydrido-aluminate(III) and sodium tetrahydridoborate(III).
Most non-metallic elements like chlorine and nitrogen form simple covalent hydrides. These are volatile compounds that are gaseous at room temperature. The exceptions are the hydrides of fluorine, HF, and oxygen, H₂O, which are liquids because of extensive hydrogen bonding. The hydrides of the more electronegative elements like chlorine and Sulphur form acidic solutions with water.
COMPOUNDS
CARBON
Carbon is a non-metal known to people for a long time under the names charcoal, soot and diamond. It occurs naturally as diamond and graphite Carbon also occurs in an impure form as coal and in the all combined state as petroleum, wood and natural gases. These carbon compounds form an important source of fuels. They are burnt to release heat and light. which may be converted to other forms of energy. Other sources that contain carbon are mineral deposits of metallic trioxocarbonates(IV), especially calcium trioxocarbonate(IV) (limestone) and magnesium trioxocarbonate(IV) (dolomite), and the carbon(IV) oxide in the air and water around us. Carbon is also an essential constituent of all living things.
Carbon is naturally present in many compounds. In addition, new useful carbon compounds are being synthesized all the time. Thus, their numbers are continually increasing.
The chemistry of carbon compounds is known as organic chemistry. However, the study of some of its compounds such as the oxides, sulphides, metallic carbides, trioxocarbonates(IV) and hydrogentrioxo carbonates(IV), is usually included in inorganic chemistry In this chapter, we shall confine our study to that of carbon itself and its inorganic compounds.
Allotropes of Carbon
The ability of carbon to exist in various forms in the same physical state is known as allotropy. Allotropy is the existence of two or more different forms of an element in the same physical state. Diamond and graphite are two allotropic forms of crystalline carbon. The others like coal, coke, charcoal, lamp-black, sugar charcoal and animal charcoal are amorphous or non-crystalline forms of carbon.
Diamond
Diamonds are found naturally in Africa, Brazil, India, the Republic of Guyana, Siberia and Venezuela. The world’s main supply of diamonds comes from south western Africa. Diamonds are the purest form of naturally occurring carbon They are found as colourless, lustreless solids which can be transformed into brilliant gems. Sometimes, they are coloured by slight traces of impurities. The diamond crystal is octahedral in shape. It is actually a giant molecule in which the carbon atoms are closely packed and held together by strong covalent bonds:
Properties
The diamond is the hardest substance known. As a result only a diamond can cut a diamond. It has a high melting point It is very dense and resistant ty high temperatures and chemical attack. It is a non conductor of electricity because there are no free valence electrons in the diamond crystal, as all of them are used in bond formation.
Uses
Since diamonds are dense and hard, they are used industrially in drills for mining, as abrasives to sharpen very hard tools, and for cutting glass and metals. They are also used as pivot supports in precision instruments and as dies for drawing wires Its high refractive index and dispersion power give it a sparkling brilliance when it is cut and polished, making it valuable as jewellery.
Artificial diamond
Artificial diamonds, suitable only for certain industrial applications, became commercially available in 1957, They are made by subjecting graphite to very high pressures and temperature for several hours, in the presence of a catalyst such as nickel or rhodium.
Graphite
Graphite occurs naturally as plumbago, an opaque black solid. It is mined mainly in Austria, China, West Germany, Republic of Korea, Madagascar, Mexico, Siberia and Sri Lanka, Like diamond, it is probably formed by the action of volcanic heat on coal deposits. The carbon atoms in graphite form flat layers. These layers are arranged in parallel, one above the other, to form a crystal lattice
Properties
Graphite is soft and Bakes easily because of its layered crystalline structure. It has a high melting point but is less dense than diamond. It is relatively inert chemically but can be oxidized to six-carbon atom organic compounds under suitable conditions. Unlike diamond, graphite is a good conductor of electricity because of the presence of mobile electrons in the crystal lattice. Mobile electrons exist since only three of the four valence electrons of each carbon atom in the graphite crystal are involved in bond formation
Uses
Graphite is an excellent dry lubricant. This is because its layered structure allows one layer to slide over another easily. Unlike oil, it is non-volatile and not sticky. It is usually used on bicycle chains and for the bearings of some motor cars. Sometimes, it mixed with oil to form a high-temperature lubricant As graphite is a good conductor of electricity and relatively inert, it is often used as electrodes in electroplating and in dry cells. A non-conductor may be made conductive by coating it with graphite
Since graphite can withstand high temperatures, it is used to line crucibles used for making high-grade steel and other alloys: A mixture of graphite and clay is used as lead in pencils. Graphite is used as a black pigment in paint, and as a neutron moderator in atomic piles.
Industrial preparation There is a great demand for graphite. It is produced industrially by heating cake in an electric, furnace to a very high temperature for about 20 to 30 hours. Air is excluded by covering the coke with sand. The graphite produced is very pure and free from grit. This process, called the Acheson process, requires a lot of energy, and is only feasible in countries with cheap electricity
Amorphous Carbon
From X-ray studies, we know that amorphous forms of carbon consist of minute crystals of graphite bound together by impurities. Thus, they are not considered as true allotropes of carbon. With the exception of coal, which is mined from natural deposits, the other amorphous forms can be prepared in various ways.
Coal
Coal was formed from the vegetation of the Carboniferous Era which was protected from complete decay by overlying water washed earth deposits. Decomposition occurred gradually under Pressure and in the absence of air. Carbon(IV) oxide. methane and water were liberated, leaving behind material that contained a very high percentage of carbon. During this process of carbonization, the vegetable material was converted in stages into peat, lignite (or brown coal), bituminous (or soft) coal, and, finally anthracite (or hard coal) which is about 95% pure carbon. Impurities present may include nitrogen, sulphur and phosphorus.
Coal is used mainly as a fuel to generate power for steam engines, factories and electric plants. It is also used for making various chemicals. In Nigeria, large amounts of coal are mined in Udi and the Milken hills of Enugu State every year. There are also extensive lignite deposits in Onitsha and Asaba
Coke
Coke is obtained by heating bituminous coal to very high temperatures (about 1300°C) in the absence of air to drive away all the volatile constituents. This process is known as the destructive distillation of coal. Coke is used mainly as a fuel. It burns with practically no smoke and leaves very little residue. It is a very important industrial reducing agent, and is used in the extraction of metals, especially iron, from their ores. It is also used in the production of gaseous fuels, like water gas and producer gas and for the manufacture of graphite, calcium carbide, silicon carbide and carbon(IV) sulphide.
Carbon black (soot)
Carbon black or soot is finely divided carbon particles produced by burning carbonaceous materials in a limited supply of air. Lamp-black is obtained from vegetable or lamp oils, while carbon black is obtained from coal gas, natural gas or fuel oils. Carbon black is used in manufacturing rubber tyres. black shoe polish, printer’s ink, typewriting ribbons and carbon paper.
Charcoal
Charcoal can be made by heating wood, nut shells. bones, sugar and even blood. Wood charcoal is the most common. It is prepared by heating wood in a limited supply of air. It contains impurities such as sulphur.
Sugar charcoal is formed when sugar is dehydrated (ie the hydrogen and oxygen it contains are removed in the form of water either by burning the sugar in a limited supply of air or by the action of concentrated tetraoxosulphate(VI) acid. It is the purest form of amorphous carbon.
Animal charcoal is produced when bones and animal refuse are heated in a limited supply of air. It contains a high percentage of calcium tetraoxophosphate(V) as impurity.
Charcoal has a very porous structure. It allows small molecules of gases and dyes to adsorb or adhere to its internal surfaces. Thus, it is a good adsorbent. particularly when activated by heating in steam, Wood charcoal is used in gas-masks adsorbing for poisonous gases. It is also used for purification of the noble gases and the recovery of industrial solvents. Similarly, animal charcoal which has the property of adsorbing colouring matter, is used in removing the brown colour from crude sugar, and in decolourizing petroleum jelly. Like coal, wood charcoal is also used mainly as a domestic fuel in Nigeria.
Carbon Fibres
Carbon fibres are produced by carefully heating libres of materials such as poly(propenonitrile) until they char to form carbon. Carbon fibres are incorporated into plastics to produce a very light but stiff and strong material.
General Properties of Carbon
All the carbon allotropes, except diamond, are black or greyish-black solids. They are odourless and tasteless. Their melting points are very high, about 3500°C They are insoluble in common solvents like water, alkalis, acids, petrol and carbon(IV)sulphide. That is why carbon deposits inside motor engines have to be removed mechanically. This known as the decarbonization of motor engines.
Chemically, carbon is not a very reactive element and most carbon compounds are stable. This is because the carbon atom has a valency of four and forms compounds with four covalent bonds. In these compounds, it does not have any lone pair of electrons and so is unreactive since it cannot function as an electron-pair donor. The stability of carbon compounds is also due to the strong carbon-carbon bond.
Carbon can form single or multiple bonds with itself and other elements such as hydrogen, nitrogen, oxygen and sulphur. Owing to the strong carbon carbon bond, carbon atoms can group together to form king chains or rings. This is known as catenation. This unique property of carbon enables it to form numerous compounds in which the molecules range from small to extremely large ones.
All the allotropes of carbon have similar chemical properties since they are all chemically identical. However, diamond and graphite are usually less reactive than amorphous carbon.
Combustion
All forms of carbon burn in excess oxygen to produce n of th bon(IV) oxide only, although the temperatures required differ
C(s) + O₂(g) → CO₂(g)
This shows that different allotropes are in fact forms of the same element. When the supply of air is limited, combustion may not be complete. Carbon(ll)oxide is formed instead of carbon(IV) oxide
2C(s) + O₂(g) → 2CO(g)
Charcoal fire: In countries like Nigeria and Ghana. charcoal is used extensively for making fires. As the charcoal burns, carbon IV oxide and carbon(II) oxide are formed at various levels inside the charcoal pot.
A charcoal pot fire receives its air supply through an aperture below the fire and also at the free surface above. Reactions at:
level C 2CO + O₂ → 2CO₂
level B CO₂+C → 2CO
level A C + 0₂ → CO₂
At level A, air is plentiful and the charcoal burns to produce carbon(IV) oxide only
At level B, the middle of the charcoal heap, the air supply is very limited. As a result, the ascending carbon(IV) oxide from level A becomes reduced to carbon(II) oxide by the carbon
At level C, the air supply is again plentiful, so that the carbon(II) oxide formed at level B is reoxidized to give carbon(IV) oxide.
Some carbon(II) oxide, however, escapes oxidation and contaminates the atmosphere. This is dangerous if the area around the fire is not well ventilated because carbon(TE) oxide is poisonous
Combination Reactions
Carbon combines directly with certain elements such as sulphur, hydrogen, calcium and aluminium at very high temperatures
C(s) + 2S(s) → CS₂(l) Carbon(IV) Sulphide
C(s) + 2H₂(g) → CH₄(g) Methane
2C(s)+ Ca(s) → CaC₂ (s) Calcium Carbide
3C(s) + 4Al(s) → AL₄C₃ (s) Aluminium Carbide
As a reducing agent
Carbon is a strong reducing agent. It reduces the oxides of the less active metals to the metals, although very high temperatures may be required in some cases. Carbon itself is oxidized to either carbon(IV) oxide or carbon(II) oxide, depending on the conditions of the reaction. At high temperatures, carbon also reduces steam to hydrogen, and carbon(IV) oxide to carbon(II) oxide.
Fe₂O₃(s) + 3C(s) → 2Fe(s) + 3CO(g)
2CuO(s) + C(s) → 2Cu(s) + CO₂(g)
H₂O(g) + C(s) → CO(g) + H₂(g)
CO₂(g) + C(s) → 2CO(g)
Reaction with strong oxidizing agents
When carbon is heated with concentrated trioxonitrate(V) acid or concentrated tetraoxosulphate(VI) acid, it is oxidized to carbon(IV) oxide.
C(s) + 4HNO₃(aq) → 2H₂O(l)+ 4NO₂(g) + CO₂(g)
C(s) + 2H₂SO₄(aq) → 2H₂O(l)+2SO₂(g)+ CO₂(g)
Destructive distillation of coal and wood
Coal is a complex mixture of compounds composed mainly of carbon, hydrogen and oxygen, with small amounts of nitrogen, sulphur and phosphorus as impurities. A wide variety of substances can be obtained from it by a process known as the destructive distillation of coal.
coal → coke + ammoniacal liquor + coal tar + coal gas
During this process, coal is heated to a very high temperature in the absence of air so that all the volatile components distil over. Some of these condense of cooling to form an almost black tar, called coal tar, and an aqueous liquid known as ammoniacal liquor. Coal tar is a mixture of more than 200 different substances which can be separated by fractional distillation. Most of these, eg. benzene, toluene, phenol and naphthalene, are used in the synthesis of important commercial products like dyes, paints, insecticides, drugs, plastics and explosives. Ammoniacal liquor is a solution of ammonia in water. It is converted into ammonium tetraoxosulphate(VI) for use as a fertilizer.
The volatile components are collected as coal gas, which usually contains about 50% hydrogen. 30% methane, 10% carbon(II) oxide and small amounts of other gases.e.g. ethene and hydrogen sulphide. Coal gas is an important gaseous fuel because it is cleaner and more efficient than coal or other solid fuels.
The non-volatile residue which is left behind wool after destructive distillation is coke, which can be used as a fuel or for other purposes. Unlike ordinary comp distillation, the coal is destroyed by this distillation process.
Wood is a complex substance like coal except that the percentage composition of the elements present in it is different. For example, wood has a higher percentage of hydrogen and oxygen but a lower percentage of carbon than coal.
Pyroligneous acid, which is the liquid fraction contains mainly ethanoic acid, propanone, methane and some other compounds.
CARBON(IV) OXIDE
Carbon forms two important oxides, namely carbon(IV) oxide, CO₂, and carbon(II) oxide, CO. The atmosphere contains about 0.03% by volume of carbon(IV) oxide. A small percentage of carbon(IV)oxide is also found in the dissolved form in water. In the combined form, it is found mainly as metallic trioxocarbonates(IV) and hydrogentrioxo carbonates(IV) in the earth’s crust, especially in limestone regions and coral reefs.
Preparation
Carbon(IV) oxide is prepared in the laboratory by the action of dilute acids on a trioxocarbonate(IV). or a hydrogentrioxocarbonate(IV). Usually, calcium trioxocarbonate(IV), in the form of marble chips. shells or “potash”, is used with hydrochloric acid or trioxonitrate(V) acid.
CaCO₃(s)+2HCl(aq) → CaCl₂(aq) + H₂O(l) +CO₂(g)
NaHCO₃(aq) + HNO₃(aq) → NaNO₃(aq) + H₂O(l) + CO₂(g)
Carbon(IV) oxide is also obtained by heating metallic trioxocarbonates(IV) (except those of sodium and potassium), or the hydrogentrioxocarbonates(IV) of sodium or potassium.
CuCO₃(s) CuO(s) + CO₂(g)
2KHCO₃(aq) → K₂CO₃(aq) + H₂O(l) + CO₂(g)
A Kipp’s apparatus is used to provide a supply of the gas whenever it is needed in the laboratory. In the Kipp’s apparatus. carbon(IV) oxide is produced by the action of dilute hydrochloric acid on marble chips.
Industrially, carbon(IV) oxide is obtained as a by-product in fermentation processes and when limestone is heated to make quicklime.
Physical Properties
1.Carbon(IV) oxide is a colourless, odourless gas with a sharp, refreshing taste.
2.It is about 1.5 times denser than air.
3.It is soluble in water. At room temperature and standard pressure, water dissolves its own volume of the gas.
4.It changes damp blue litmus paper pink because carbon(IV) oxide dissolves in water to yield trioxocarbonate(IV) acid.
5.On cooling, it readily liquefies and solidifies (-78 °C) to form a white solid known as dry ice.
Chemical Properties
Reaction with water: Carbon(IV) oxide is not very active chemically. It dissolves in water to form trioxocarbonate(IV) acid (soda water). This is a weak, dibasic acid which ionizes slightly.
a)CO₂(g) + H₂O(l) ⇋ H₂CO₃(aq)
b)H₂CO₃(aq) + H₂O(l) ⇋ H₃O⁺(aq) + HCO₃⁻(aq)
c)HCO₃⁻(aq) + H₂O(l) ⇋ H₃O⁺(aq) + CO₃²⁻ (aq)
On heating, trioxocarbonate(IV) acid decomposes to form water and carbon(IV) oxide,
Reaction with alkalis
It reacts directly with alkalis to yield trioxocarbonates(IV).
CO₂(g) + 2NaOH(aq) → Na₂CO₃(aq) + H₂O(l)
Ionically.
CO₂(g) + 2OH⁻(aq) → CO₃²⁻ (s or aq) + H₂O(l)
In the presence of excess carbon(IV) oxide, the trioxocarbonates(IV) formed react with the carbon(IV) oxide to produce hydrogentrioxocarbonates(IV).
Na₂CO₃(aq) + H₂O(l) +CO₂(g) → 2NaHCO₃(aq)
lonically,
CO₃²⁻ (s or aq) + H₂O(l) + CO₂(g) → 2HCO₃⁻(aq)
Solutions of alkalis absorb carbon(IV) oxide readily and are frequently used to remove it from a mixture of gases.
Reaction with burning magnesium
Carbon(IV) oxide does not burn, nor does it support the combustion of most substances. The intense heat produced by burning magnesium, however. decomposes carbon(TV) oxide to release oxygen for the further oxidation of magnesium. The products of the combustion are carbon deposits and white magnesium oxide ash.
CO₂(g) + 2Mg(s) → 2MgO(s) + C(s)
Reaction with red-hot carbon
If the gas is passed over red-hot carbon, it is reduced to carbon(II) oxide. This reaction is reversible and is of great commercial importance.
CO₂(g) + C(s) ⇋ 2CO(g)
Test for carbon(IV) oxide
Bubble the unknown gas through lime water (calcium hydroxide). If the gas is carbon(IV) oxide, the lime water turns milky due to the precipitation of insoluble calcium trioxocarbonate(IV)
Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
Continue bubbling more of the gas through the solution. The milkiness should disappear leaving a clear solution. This is because carbon(IV) oxide reacts with insoluble calcium trioxocarbonate(IV) to form soluble calcium hydrogentrioxocarbonate(IV)
CaCO₃(s) + H₂O(l) + CO₂(g) → Ca(HCO₃)₂(aq)
Finally, heat the clear solution. It should become milky again due to the decomposition of soluble calcium hydrogentrioxocarbonate(IV) to form insoluble calcium trioxocarbonate(IV)
Ca(HCO₃)₂(aq) → CaCO₃(s) + H₂O(l) + CO₂(g)
*************************Uses
Solid carbon(IV) oxide (ie. dry ice) is used as a refrigerant for perishable goods, e.g. ice-cream. It sublimes on warming and provides a lower temperature. Gaseous carbon(IV) oxide is used to preserve fruits. Carbon(IV) oxide is also used as a coolant in nuclear reactors.
CARBON(II) OXIDE
Carbon) oxide, CO, is produced by the incomplete combustion of carbon compounds, such as octane, C₈H₁₈. found in petrol.
2C₈H₁₈(l) +17O₂(g) → 16CO(g) + 18H₂O(l)
Carbon(II) oxide occurs in traces as an impurity in the atmosphere. The percentage present may be higher in cities where the gas is released in the exhaust fumes of motor cars, and in industrial areas due to the combustion of fuels. Carbon(II) oxide is a poisonous gas. As little as 0.5% of it in the air may cause a person to die. Since the gas has no colour or odour, its presence is difficult to detect, so it is very dangerous.
Preparation
Carbon(II) oxide is prepared from carbon(IV) oxide by passing the latter through red-hot carbon as shown in fig. 7.13. Most of the carbon(IV) oxide gets reduced to carbon(II) oxide. Unchanged carbon(IV) oxide is removed when the mixture of gases passes through concentrated sodium hydroxide. The pure carbon(II) oxide is collected over water.
CO₂(g) + C(s) → 2CO(g)
**********Carbon(II) Oxide can be ***********
Physical properties
1.Carbon(II) oxide is a colourless, tasteless and odourless gas
2.It is insoluble in water, but dissolves in a solution of ammoniacal copper(I) chloride.
3.It is slightly less dense than air
4.It is neutral to litmus
Chemical properties
As a reducing agent: Carbon(II) oxide is a strong reducing agent. It reduces some metallic oxides to metals and is itself oxidized to carbon(IV) oxide.
PbO(s) + CO(g) → Pb(s) + CO₂(g)
Carbon (II) oxide also reduces iodine(V) oxide to iodine, and steam to hydrogen.
I₂O₃(s) + 5CO(g) → I₂(s)+ 5CO₂(g)
H₂O(g) + CO(g) → H₂(g) + CO₂(g)
Combination reactions
With oxygen: Carbon(II) oxide burns in air with a faint blue flame forming carbon(IV) oxide.
2CO(g) + O₂(g) → 2CO₂(g)
With haemoglobin Carbon(II) oxide is a poisonous gas since it combines with haemoglobin in the red blood cells to form a stable compound — carboxyhaemoglobin, COHb
CO+Hb → COHb
This stable compound prevents the haemoglobin from transporting oxygen in our body. A person will die from lack of oxygen when one-third of the haemoglobin in the body is combined with carbon(II) oxide.
Test for carbon(II) oxide
Bubble some of the unknown gas through a test-tube containing lime water. Next, apply a lighted splint to a test-tube containing the unknown gas. Note what happens. Then add some lime water to the test-tube and shake. If the gas is carbon(II) oxide, it will burn with a blue flame and turn lime water milky after burning but not before burning
Uses
Carbon(II) oxide is used in the extraction of metals from their ores. It is also an important constituent of gaseous fuels like producer gas and water gas.
Fuel Gases
Producer gas
Producer gas is a mixture of nitrogen and carbon (II) oxide, prepared by passing a stream of air through red-hot coke. The oxygen in the air oxidizes the coke to carbon(II) oxide, with the liberation of a lot of heat while the nitrogen is unchanged. Some carbon(IV) oxide may be formed but this is usually reduced by the hot coke to carbon(II) oxide.
O₂(g) + N₂(g) + 2C(s) → 2CO(g) + N₂ + heat
Producer gas
Producer gas has a low heating power because it contains about 67% non-combustible nitrogen and 33% carbon(II) oxide. However, it is inexpensive and is widely used to heat furnaces, retorts (in the manufacture of zinc and coal gas) and limekilns. It is also a source of nitrogen for the manufacture of ammonia (Haber process).
Water gas
Water gas is a mixture containing equal volumes of hydrogen and carbon(II) oxide, prepared by passing steam over white-hot coke at 1000°C
C(s)+ H₂O(g) → CO(g) + H₂(g)
During the process, the coke quickly cools to a temperature too low for reaction if heat is not supplied externally. Industrially, producer gas and water gas are made in the same plant, known as the producer. by passing air and steam alternately through the heated coke. The heat produced when producer gas is formed is sufficient for water gas formation. Both the hydrogen and the carbon(II) oxide in water gas burn in air releasing a lot of heat. This makes water gas an important industrial fuel. However, it has too high a carbon(ll) oxide content for domestic use. Water gas is also an industrial source of hydrogen and other organic compounds, eg. methanol and butanol.
TRIOXOCARBONATES(IV)
Trioxocarbonate(IV) acid is a dibasic acid. It forms two series of salts:
-the normal trioxocarbonates(IV), and
-the acidic hydrogentrioxocarbonates(IV).
Trioxocarbonates(IV) are formed naturally when trioxocarbonate(IV) acid, formed when carbon(IV) oxide dissolves in water, reacts with free metals, metallic oxides or other dissolved salts. Metallic trioxocarbonates(IV) are usually found as natural ores or deposits.
Preparation
Preparation of soluble trioxocarbonates(IV)
Of the common trioxocarbonates(IV), only sodium, potassium and ammonium trioxocarbonates(IV) are soluble in water They are prepared in the laboratory by bubbling carbon(IV) oxide through a solution of the corresponding alkali
2KOH(aq) + CO₂(g) → K₂CO₃(aq) + H₂O(l)
Since sodium and potassium trioxocarbonates (IV) are not decomposed by heating, they can also be prepared by heating the corresponding hydrogentrioxocarbonates(IV).
2KHCO₃(s) → K₂CO₃(s) + H₂O(l) + CO₂(g)
Preparation of insoluble trioxocarbonates(IV)
The insoluble metallic trioxocarbonatestV) can be prepared as precipitates by adding a solution of sodium trioxocarbonate(IV) or sodium hydrogentrioxocarbonate(IV) to a solution of the corresponding metal salt
CaCl₂(aq) + Na₂CO₃(aq) → CaCO₃(s) + 2NaCl(aq)
CaCl₂(aq) + 2NaHCO₃(aq) → CaCO₃(s) + 2NaCl(aq) + H₂O(l) + CO₂(g)
2AgNO₃(aq) + Na₂CO₃(aq) → Ag₂CO₃(s) + 2NaNO₃(aq)
ORGANIC CHEMISTRY 1
WHAT IS ORGANIC CHEMISTRY?
The term, organic chemistry originally meant the chemistry of compounds obtainable from plants and animals-living organisms. The reason for this belief was because they believed that to produce organic compounds you needed a vital force.
The idea of a vital force was put in doubt in 1927/28 when Frederick Wohler, a German chemist. produced urea (produced by man in urine) from ammonium cyabate (NH₄⁺CNO⁻, a pure inorganic compound).
A more serious blow to the vital force theory was the synthesis of acetic acid (CH₃COOH) by Kolbe in 1845 from its elements. The preparation of methane (CH₄) by Berthelot in 1856 confirmed that the vital force theory had no chemical basis.
CS₂ + H₂S → CH₄
Today, organic chemistry is effectively defined as the chemistry of carbon together with a few other elements like. H, N, O, S. Today, there will over 3000 organic compounds and more compounds are added to the list either by syntheses in the laboratory or by discovery in nature.
The uses of organic compounds are innumerable. Some are eaten as food, others are worn as clothing, they include the wonder drugs, the most deadly poisons and the most effective fertilizations. The ultimate source of organic compounds is the sun. Carbon combines with chlorophyll in green leaves in the presence of sun’s energy to produce the basic hydrocarbon. These are then acted upon by heat and pressure to produce the more complex organic compounds.
The Apparent Unique Nature of Carbon
It is easy to explain the unique of carbon which ha resulted in giving it a chemistry all to its own among the 100 odd elements in the periodic table.
1.The valency of carbon. Carbon has the following configuration: 1s² 2s¹ 2px¹ 2py¹ 2pz¹
It forms mainly covalent bonds and after covalency it has neither vacant orbital nor I one pair B. Thus many carbon compounds are chemically stable compared with analogues compounds of other elements.
2.The second principal quantity number of carbon has no d’ orbital. Thus when carbon has formed four bonds, there are no other orbital available for bonding.
3.The bond between carbon and hydrogen is almost non-polar. Thus, hydrogen atoms attached to carbon do not weaken carbon-carbon bonds. The electronegativity of C is 2.5, hydrogen 2.1 and fluorine is 4.0. So the fluorine will weaken the carbon-carbon bond in fluoroethane
4.The types of orbital hybridization available to carbon. There are three types of hybridization open to carbon namely Sp³. Sp² and Sp hybridization. These give rise to a variety of neutral compounds having single, double and triple bonds
5.Delocalisation or continuous 𝝅-bonding. This occurs in carbon compounds than in any compounds of other elements. This phenomenon assures that six-membered ring carbon compound with delocalized orbitals are particularly stable. Compare benzene and borazine which is chemically less stable than aromatic carbon compounds.
OCTANE NUMBER **can’t seee the left edge ****
This is the rating of the performance of a petrol in internal combustion engine. A poor quality fuel ds to knock or explode unevenly and prematurely Specially in a high compression engine 2, 2, 4 ethylpentane (Iso-octane) is a good fuel since it smoothly and does not cause knocking, it is
an arbitrary octane number zero. The octane pamber of any fuel is the percentage
knocking characteristics as the fuel when comparisons are made in a standard engine. The octane member of a fuel is raised by the presence of branched chain hydrocarbons and aromatic compounds. Ethanol and methanol when added to some petrols improve their performance. Again tetracthyl lead(iv), Pb(C₂H₅) in small quantities greatly increases the octane number of a fuel 0.03% increase by weight may raise the octane number by 15 or 20 units. It should be remembered that lead oxides from exhaust fumes are bad pollutants
Nowadays, most of these substances can be synthesized from inorganic materials in the laboratory, Many other similar compounds which do not have any connection with living organisms are also known. We now define organic chemistry as the chemistry of carbon compounds. Apart from a few compounds such as the oxides, the trioxocarbonates(IV) and the metallic carbides, which are conventionally regarded as inorganic, all other carbon compounds are grouped together in this separate branch of:
●their large number:
●their many common features; and
●the great medical, biochemical and economic importance of many of them.
VARIETY OF ORGANIC COMPOUNDS
Organic compounds are made up of:
●the main element, carbon,
●hydrogen and oxygen which are usually present, and
●elements such as nitrogen, the halogens, phosphorus, sulphur and some metals which are sometimes present
The presence of numerous organic compounds is due to the following properties of carbon:
1.The exceptional ability of carbon atoms to catenate, ie. to combine with one another to form straight chains, branched chains or ring compounds containing many carbon atoms
2.The ease with which carbon combines with hydrogen, oxygen, nitrogen and the halogens.
3.The ability of carbon atoms to form single, double or triple covalent bonds.
4.The bond energy for carbon to carbon single bond is high. The heat energy needed in kilojoules to break one mole of bonds.
C—C = 846, N—N = 163, O—O = 146, Si—Si = 175
We can illustrate the ability of carbon to form a variety of compounds by showing how five carbon atoms can join together to form some compounds.
We have only shown five of the possible compounds that can be formed by using five carbon atoms. Many more compounds can be formed using various combinations of straight chains, branch chains, rings, multiple bonds and other atoms such as oxygen and the halogens. This is why there are so many organic compounds.
CHARACTERISTIC FEATURES OF ORGANIC COMPOUNDS
Covalent nature: Carbon atoms form stable covalent bonds with one another. A carbon-carbon single covalent bond has an energy of 346 kJ mol. This high energy indicates a strong bond. Most organic compounds are stable because of the strong carbon-carbon bonds. Since they have a covalent nature, they do not ionize in solution and are non-conductors of electricity.
Polarity and solubility of non polar compounds: Carbon-hydrogen bonds are, non-polar, like the carbon carbon bonds. This is because of the almost equal electronegativities of the two elements. Most organic compounds are non-polar unless the compounds consist of very electronegative elements like chlorine or groups like the hydroxyl group.
Since most organic compounds are non-polar, they cannot form bonds with water molecules. So most organic compounds are insoluble in water. For example, petrol, kerosene and paraffin oil do not dissolve in water. If an organic compound contains polar groups, hydrogen bonds can form between the polar groups in the molecules of the organic compound and the water molecules. This enables the compound to dissolve in water. For example, an ethanol molecule contains a hydroxyl group which is polar, so it is soluble in water. Non-polar substances are held together only by weak intermolecular forces such as the van der Waals forces, and so they can intermingle easily. This is why most organic compounds dissolve only in non-polar solvents like benzene or ether.
Low melting and boiling points: Organic compounds generally have lower melting and boiling points than inorganic compounds. This is because these compounds possess relatively weak intermolecular bonds which can be easily broken by heat energy. Many of them (mainly those with low relative molecular masses) tend to be volatile and boil at temperatures below 300°C.
Thermal instability: Many organic compounds are thermally unstable, decomposing into simpler molecules when heated to temperatures above 500°C. However, this property is sometimes of commercial importance as in the cracking of petroleum.
Flammability: Most organic compounds are flammable and burn exothermically in a plentiful supply of air to yield carbon(IV) oxide and water. Thus, most fuels such as wood, coal, oil, petrol and natural gas are organic and their combustion provides our main source of heat energy.
Reactivity: Reactions involving organic compounds tend to be much slower than the ionic reactions commonly encountered in inorganic chemistry. They usually require heating, thorough mixing and catalyst to speed up the reactions
TERMS IN ORGANIC CHEMISTRY
In the study of organic chemistry, several terms are used frequently. Some of these important terms are introduced and explained here to facilitate better understanding in the subsequent sections.
HYBRIDIZATION OF ORBITALS
Hybridization is the combination or mixing of a given number of orbitals in an atom to produce the same number of new orbitals that are different in shape and better disposed for chemical bonding. The new orbitals are called hybrid orbitals. See hybridization in carbon atom.
HYBRIDIZATION IN CARBON ATOM
Carbon with an electronic configuration of 1s² 2s¹ 2px¹ 2py¹ 2pz¹ has two unpaired electrons. But it shows a covalency of four instead of two. To account for its tetravalence it is proposed that the ground state carbon first absorbs energy to become excited before bonding takes place. The energy absorbed is used to promote a 2s-electron into the vacant 2p-orbital. An excited carbon atom thus has the electronic configuration of 1s² 2s¹ 2px¹ 2py¹ 2pz¹. The 2s orbital is spherical and of lower energy than 2p-orbitals, which are right angles to one another. The shape of tetrahedral structure of methane tetrachloromethane ele is accounted for by assuming that before the overlap of the orbital of the excited carbon atom by four hydrogen or for chlorine atoms to form methane tetrachloromethane there is rearrangement of the unpaired orbitals. The rearrangement involves an equalization in energy of the four unpaired orbitals and their spreading out. This termed hybridization.
Hybridization is the mixing of two or more orbitals of the same principal quantum number to give new sets of orbitals, which are exactly equivalent. The new orbitals are known as hybrid orbitals. Hybridization can be Sp³, Sp² or Sp.
Sp³ hybridization
When one S-orbital hybridize with three p-orbitals of an excited carbon atom, SP³ hybridization is formed. After spreading out, the unpaired orbitals are aligned at 109° away from each other. They point to the cornets of a regular tetrahedron with carbon atom at the centre of the tetrahedron. The overlap of each hybrid orbital with the orbital of a hydrogen atom or chlorine atom results in a methane and tetrachloromethane, which are tetrahedral in shape.
Sp² hybridization
When only 2 of the 3 unpaired P-orbitals in an excited carbon atom hybridize with the unpaired 2s-orbitals, SP² hybridization is said to have taken place. It results in three hybrid orbitals directed 120° away from each other on the same plane. The third P-orbital which does not take part in the hybridization remains at right angles to the plane of the hybrid orbitals.
SP hybridization
A triple bond is formed when only one unpaired P-orbital and 2s¹ orbital of an excited carbon atom hybridize. This is termed SP hybridization. The two hybrid orbitals spread out fully, so that one is 180° away from the other. The two unhybridized P-orbitals remain at right angles with the hybrid one.
Homologous series
The numerous organic compounds can be grouped into a comparatively small number of series or families of compounds known as homologous series. The simplest series of compounds in organic chemistry is the alkanes. We shall study this series to illustrate what a homologous series means.
The alkanes are a series of hydrocarbons with a general molecular formula of CₙH₂ₙ₊₂ where n is a whole number with a value of one or more
Each individual member of the alkane series differs from the preceding or the following member by one carbon atom and two hydrogen atoms, ie. –CH₂– group. Such a family of compounds is known as a homologous series and each individual member is referred to as a homologue,
A homologous series is a family of organic compounds which follows a regular structural pattern, in which each successive member differs in its molecular formula by a –CH₂– group.
Other homologous series include the alkenes, CₙH₂ₙ, the alkanols, CₙH₂ₙ₊₁OH, and the carboxylic acids, CₙH₂ₙ₊₁COOH. Homologous series have the following common characteristics:
General molecular formula: All members share a general molecular formula, i.e. CₙH₂ₙ₊₂ for the alkanes, CₙH₂ₙ for the alkenes, and so on.
Difference between successive homologues: Each successive member in such a series differs in its molecular formula by the addition of a –CH₂– group, and in its relative molecular mass by an increase of 14.
Physical properties: The physical properties of the members change gradually as the number of carbon atoms per molecule increases. For example, the boiling points of the alkanes increase down the series, so that the first four members are gases at room temperature and standard pressure; members with five to seventeen carbon atoms per molecule are volatile liquids, while the higher members are wax-like solids. Similarly. the melting points and densities of the alkanes also increase, while their solubility in water decreases down the series
Chemical properties: The members show similar chemical properties. For example, the alkanes are fairly unreactive under ordinary conditions. They burn in air, forming carbon(IV) oxide and water, and undergo substitution reactions with other substances such as halogens.
General methods of preparation: All members can usually be prepared by using the same general methods, e.g. alkanes can be prepared by the action of hot soda lime on the appropriate sodium salt of an acid
Alkyl Groups
Alkyl Groups: Many homologous series can regarded as being derived from the alkanes by substitution of one or more of the hydrogen atoms by other elements or groups. The univalent group which formed from an alkane by the loss of a hydrogen a is known as the alkyl group. Thus, the compounds is formed by substitution can be considered as be made up of the alkyl group and the substituent group For example, chloromethane, CH₃CI, is composed the methyl group, –CH₃– (obtained from methane CH₄, by the loss of a hydrogen atom) linked to e substituent chlorine atom. –Cl
The general term alkyl group includes all groups derived from the alkanes by the loss of a hydrogen atom. Alkyl groups have a general formula of CₙH₂ₙ₊₁. They are named after the parent allanes by replacing the ending -ane by -yl. Alkyl groups are given the general symbol. R Sometimes, R may stand for more complex group than just simple alkyl groups.
Functional groups: The alkyl group of a compound is fairly inert chemically because of the stability of the carbon-hydrogen bonds. The chemical reactivity of an alkyl compound is determined mainly by the substituent group. These groups are referred to as the functional groups, eg the hydroxyl group –OH, the amino group, –NH, the carboxyl group, –COOH, and the double covalently bonded carbon atoms, C═C. Each functional group has its own characteristic properties. When two or more functional groups occur in one molecule, the properties of one are often modified or influenced by the presence of the others. Thus, the presence of the functional group or groups determines the chemical properties of a homologous series.
A functional group is an atom, (a radical group of atoms) or a bond common to a homologous series, and which determines the main chemical properties of the series.
Effect of the alkyl and functional groups: The functional group determines the basic chemistry of a compound, while the alkyl group affects the physical properties of a compound. For example, the polar hydroxyl group in the alkanols promotes solubility in water but the non-polar alkyl group opposes For all alkyl groups larger than C₂H₅–, this opposing effect is sufficient to greatly limit the solubility of the compound in water.
Saturated and unsaturated compounds
If an organic compound contains atoms joined only by single covalent bonds, the compound is said to be saturated. The alkanes are said to be saturated hydrocarbons.If an organic compound contains carbon atoms joined by double or triple covalent bonds, the compound is said to be unsaturated.
Hydrocarbons possessing a set of carbon-carbon double covalent bonds, ⤫C═C⤫, in their carbon chains form the homologous series called alkenes while those possessing a set of carbon-carbon triple covalent bonds, –C═C–, in their carbon chains form the alkyne series. Ethene and ethyne are the first members of the alkenes and alkynes respectively. Both these series can be considered as being derived from the saturated alkanes by the removal of hydrogen atoms and the subsequent introduction of double and triple bonds respectively in their carbon chains. The carbon-carbon double and triple covalent bonds represent the functional groups in these series because the availability of electrons in these multiple bonds makes the unsaturated compounds chemically more reactive. Compounds containing double or triple bonds between a carbon atom and an atom of another element can also be said to be unsaturated.
Formulae of organic compounds
Empirical formula: The simplest formula of a compound is called its empirical formula. It indicates the relative numbers of each kind of atom in a molecule of a substance. It is found by determining the percentage composition of the substance by quantitative analysis.
Molecular formula: A more useful formula than the empirical formula is the molecular formula of a substance. It indicates the actual numbers of each kind of atoms in a molecule of a substance. The molecular formula is deduced from the relative molecular mass of the substance and its empirical formula.
Structural formula: In inorganic chemistry a molecular formula is informative enough to distinguish one substance from another. This is not the case in organic chemistry where the molecular formula may represent more than one substance. A more informative formula called the structural formula is often used to represent a particular organic substance. Such a formula indicates how the atoms are arranged within the molecule of a substance. The structural formula of ethanoic acid can be represented as follows:
The formula shows which atoms are linked together and how they are linked by using conventional symbols. The structural formula can also be written in a condensed form where the alkyl and functional groups present in the molecule are shown and arranged in the correct order. Ethanoic acid can be represented as CH₃COOH
This condensed structural formula is usually preferred because it indicates clearly the functional groups present in the substance. The structural formula can be determined through various spectroscopic methods.
The structural formula is usually shown as planar for convenience and does not imply a flat molecule. A true molecular representation of an alkane, for example, should show the actual arrangement of the atoms in space and the angles between the bonds Thus, the methane molecule, CH,, should show a central carbon atom joined to each hydrogen ato by covalent bonds which are distributed tetrahedrally around the carbon atom, i.e. the bonds are at an angle of 109° 28′ to one another. This is known as the spatial or three dimensional representation of the methane molecule. Fig. 30.2 shows the spatial representations of the molecules of several common organic compounds. Methane, ethane and propane show tetrahedral structures while ethene is planar and ethyne linear.
THE IUPAC NOMENCLATURE FOR ALIPHATIC COMPOUNDS
The International Union of Pure and Applied Chemistry (IUPAC) has put forward a system nomenclature which relates the name of the compound to its molecular structure. In this system, every name consists of a root, a suffix and as many prefixes necessary.
Root
The root is generally an aliphatic hydrocarbon. All aliphatic compounds are regarded as being derived from this root hydrocarbon by:
●the replacement of hydrogen atoms by alkyl or functional groups;
●the introduction of multiple bonds (i.e. double and triple covalent bonds).
The systematic name of a compound is formed from the root hydrocarbon by adding a suffix and prefixes to denote the substitution of the hydrogen atoms.
Table 30.4 gives the common functional groups which usually replace the hydrogen atoms in the root hydrocarbon, together with their names when they are used as suffixes or prefixes.
Suffixes
A suffix is added to the root to indicate the presence of the principal substituent, which is usually also the principal functional group in the molecule. Compounds having the same functional groups, such as those belonging to the same homologous series, would carry a common suffix at the end of their names. The following are some examples:
Alkanes: The names of alkanes end with -ane, e.g. Methane, CH₄, ethane, C₂H₆, and propane, C₃H₈
Alkenes: The members of the alkene series are formed from the alkanes by the removal of two hydrogen atoms and the introduction of a double bond in the carbon chain. They are named after the corresponding alkanes by changing the -ane ending to -ene, e.g. C₂H₄, is ethene. C₃H₆, is propene and C₄H₈, is butene.
Alkynes: Each member of this series is formed by the removal of four hydrogen atoms and the introduction of a triple bond in the appropriate alkane molecule. They are named by replacing the -ane ending by -yne. e.g. C₂H₂, is ethyne and C₃H₄, is propyne.
Alkanols: The members of this series, ROH, are named after the corresponding alkanes by replacing the -e ending with -ol, eg. CH₃OH is methanol and C₃H₇OH is propanol.
Alkanoic acids: Also known as carboxylic and organic acids, RCOOH, the members of this series are named by replacing the -e ending in the corresponding alkanes by -oic acid, c.g. CH₃COOH is ethanoic acid, C₂H₅COOH is propanoic acid and C₃H₇COOH is butanoic acid.
Esters: Esters, RCOOR’, are formed when a carboxylic acid reacts with an alkanol. They have a general formula of RCOOR’, where RCO- is the group derived from the acid and -OR’ is the group derived from the alkanol. In naming an ester, the alkyl group, R’, is named first, followed by the name of the acid group with a -oate ending as the suffix. For example, HCOOCH, is methyl methanoate, CH₃COOCH, is methyl ethanoate and CH₃(CH₂)₂COOC₂H₅ is ethyl butanoate.
Amides: Members of this series, RCONH₂, have an -amide ending in their names, instead of the -e in the corresponding alkanes. For example, CH₃CONH₂ is ethanamide and C₂H₅CH₂CONH₂ is butanamide.
Alkanals or (aldehydes): The members of this series, RCHO, are named by replacing the -e ending in the corresponding alkanes by -al. For example, HCHO is methanal, CH₃CHO is ethanal and C₄H₉CHO is pentanal
Alkanones or (ketones): The members of this series, RCOR’ have an -one ending in their names, instead of thee in the corresponding alkanes, eg, CH₃COCH₃ is propan-2-one and CH₃COC₂H₅, is butan-2-one.
Amines: The members of this series, RNH₂, are named by adding the -amine ending to the alkyl group, eg CH₃NH₂ is methylamine and C₂H₅NH₂ is ethylamine.
Prefixes
Cyclic compounds can be indicated by adding the prefix cyclo- to the names of the corresponding aliphatic compounds. e.g. cyclohexane and cyclopentene. Prefixes are also used to indicate the presence of substituted alkyl or functional groups other than the principal group, as well as the positions of these substituents in the carbon chain.
Alkoxy prefix: In the case of ethers, which have the general formula of R-O-R’, where R and R’ may be the same or different alkyl radicals, the prefix alkoxy is used. This prefix. –OR’, is composed of the name of the simpler alkyl group in the molecule with the -yl ending replaced by -oxy. The alkoxy prefix is then followed by the name of the corresponding alkane of the other alkyl group in the molecule. For example, CH₃OC₂H₅ is methoxyethane and C₂H₅OC₂H₅ is ethyloxyethane.
Rules for naming more than one prefix: When more than one of the same substituent group is present. the multiplying prefixes, such as di- for two, tri- for three and tetra- for four are used. If more than one prefix is needed, they are placed in alphabetical order. Multiplying prefixes do not affect this order. For example, CICH₂CHBr₂, is bromochloroethane and CICH₂CHBr₂, is 1,1,dibromo-2-chloroethane.
Numbering of the carbon atoms
The positions of the substituent groups and the multiple bonds in the carbon chain of a compound are indicated by the number of the carbon atom or atoms to which they are attached. The IUPAC convention is to number all the carbon atoms in the longest chain starting from the end which is the closest to the branch chain or other modifications of the simple alkane structure. This is done so as to give:
●the lowest possible number to the group cited by the suffix, and then
●the lowest possible individual numbers to the groups cited as prefixes.
Numbers (especially 1) are often omitted when the structure can be deduced without them. e.g. butanone can only be CH₃CH₂COCH₃.
Basic rules for naming organic compounds
Aliphatic organic compounds can be named by following these basic rules.
1.Take the longest continuous carbon chain as the root hydrocarbon and name it according to the number of carbon atoms it contains, adding the appropriate suffix to indicate the principal substituent group.
2.Number the carbon atoms in the root hydrocarbon from the end which will give the lowest number to the suffix, and then the prefix(es).
3.Indicate the other substituents by prefixes preceded by numbers to show their positions on the carbon chain.
4.When two different alkyl groups are substituted to one organic compounds, they should be named based on the one that came first alphabetically e.g. 4-ethyl-2-methylHexane
STEREOCHEMISTRY
Stereochemistry is the study of structure in three dimensions. Here we are interested in the phenomena of isomerism.
Isomerism
If we study the structural formula of butane or other higher hydrocarbons of the alkane series, we will observe that it is possible to arrange the atoms in the molecule in more than one way. This means that it is possible to have two or more different structural Arrangements for the same molecular formula. For example, the four carbon atoms and ten hydrogen alors in the butane molecule can be linked in two different ways which will satisfy the valencies of carbon and hydrogen.
This would mean that there are two compounds (shown above) with the molecular formula C₄H₁₀. This has been supported by experimental evidence which shows that there are two compounds with different physical properties (e.g the boiling point of one is 0.6°C, while that of the other is 12°C) but with the same molecular formula of C₄H₁₀. Such a phenomenon is known as isomerism.
Note: The branched compound 2-methylpropane (isobutane) has the lower boiling point than normal butane due to the presence of weaker van der Waals forces between the molecules.
Isomerism is the existence of two or more compounds (known as isomers) with the same molecular formula but different molecular structures
Isomerism is a very common feature in organic chemistry. As the number of carbon atoms in a molecule increases, the number of isomers also increases. For example, pentane has the following three possible isomers.
Hexane, C₆H₁₄, has five possible isomers, while calculations show that there are 75 isomers for decane,C₁₀H₂₂, and up to 366319 isomers for eicosane, C₂₀H₄₂. Generally, isomers with the same molecular formula and belonging to the same homologous t series tend to have similar chemical properties (as they have the same functional group), but slightly different physical properties as a result of their structural differences. However, it is also possible to have isomers with the same molecular formula but belonging to different homologous series. For example, both ethanol and methoxymethane (dimethyl ether) have the same molecular formula of C₂H₆O, but belong to the alkamal and ether series respectively. Such isomers usually have different physical as well as chemical properties because of their different structural formulae and functional groups respectively
Thus, ethanol is a liquid at room temperature and it reacts readily with phosphorus(V) chloride due to the presence of the hydroxyl group. -OH Methoxy methane, which has a lower boiling point, exists as a vapour at room temperature and does not react with phosphorus(V) chloride due to the absence of the hydroxyl group
Geometric Isomerism
We can write the structural formula of butane in two ways, since each carbon atom joined by the single covalent bond has two different atoms/groups attached to it. However, only one form of butane exists because there is free rotation about the C–C single bond. The situation differs if double bonds are present instead of single bonds. Thus at any given moment, a compound like but-2-ene may exist in two forms as represented by the following structural formulae
In but-2-ene, the presence of the double bonil between the carbon atoms hinders free rotation. So the two forms, cis- and trans are locked in shape, giving rise to geometric isomerism. Geometric isomerism is the existence of compounds with the same molecular formula but are not identical because of different spatial arrangement of the compound atoms.
Geometric isomers have similar chemical properties, but their physical properties are different e.g, the trans-form of 1,2-dichloroethene boils at 48°C while the cis-form boils at 60 °C. Geometric isomers are compounds with the same molecular formula but a different orientation in space.
Geometric isomerism is common among alkenes. An alkene where each carbon atom joined by a double bond is attached to two different atoms or groups can exist in the cis- and trans- forms.
NOTE: When two heavy or large groups (groups with large molecular masses) are on the same side of the double bond, the molecule is said to have cis configuration. When two such groups lie on the opposite side of the double bond, the molecule is said to possess a trans configuration
Optical Isomerism
Optical isomerism is the existence of two or more compounds with the same molecular formula but with different configurations, and because of molecular asymmetry they rotate plane polarised light. Consider white light. This vibrates in many directions (planes), but when passed through a Nicolprism, the light vibrates in one plane only and is said to be plane polarised. A compound that rotates plane polarised light is said to be optically active. For a compound to be optically active, it must have a carbon atom which is substituted by four different groups e.g Lactic acid.
The compound and its mirror images must not be superimposable on each other. When the compound rotates the plane polarised light to the right, it is said to be dextro-rotatory and is represented by d- or (+). When the light is rotated to the left, the compound is Laevo-rotatory and is designated L– or (–).
CLASSIFICATION OF ORGANIC COMPOUNDS
Organic compounds can be classified into aliphatic and aromatic compounds according to their molecular structures, i.e. the arrangement of atoms in the molecules.
Aliphatic Compounds
Compounds whose molecules are composed of chains of carbon atoms are known as aliphatic compounds. There is no limit to the number of carbon atoms in a given chain. Often, a given carbon chain may even have one or more branches. Some examples of aliphatic compounds are:
●pentane (a straight chain compound), and
●2-methylbutane (a branched chain compound)
Such straight and branched chain aliphatic compounds are called acyclic compounds.
In the actual molecular structures, the carbon chains are not in truly straight lines as is conveniently represented on paper. Instead, the chains are in zig-zag lines because of the tetrahedral nature of the carbon bonds. Sometimes, the end carbon atoms of an open aliphatic chain can also join together to form a closed system or ring as in cyclopropane and cyclohexane. Such compounds are known as cyclic compounds.
Aromatic Compounds
Aromatic compounds are a special class of compounds whose structures are based on the structure of benzene, C₆H₆ – a 6-carbon ring compound. They can also be described as benzene like compounds. Some of them are derivates of benzene eg aniline (phenylamine) and phenol. Others are not derivatives of benzene eg. pyrrole, thiophen and furan but related to benzene in satisfying the conditions for aromaticity namely:
i) Must be cyclic (ring form)
ii) Must be planar
iii) Must have a system of (2n+4) 𝝅-electrons or 6, 10, 14 electrons
HYDROCARBONS
Hydrocarbons are among the simplest organic compounds because they are composed only of two elements, namely carbon and hydrogen. All hydrocarbons have the molecular formula of CₓHᵧ where x and y are positive whole numbers. Some examples are methane, CH₄, propane, C₃H₈, and benzene, C₆H₆
Hydrocarbons are classified into two main groups – the aliphatic hydrocarbons and the aromatic hydrocarbons, according to their structure.
Sources of Hydrocarbons
The natural sources of hydrocarbons are coal, natural gas and petroleum. These are often known as fosil fuels because they are:
●the remains of plants and animals that died millions of years ago, and
●used mainly as fuels, that is burnt to release heat or other forms of energy.
Coal is a solid fuel. petroleum is a dark viscous liquid fuel and natural gas is a gaseous fuel.
Petroleum
Crude oil or petroleum is the most important source of fuel nowadays. Petroleum is first fractionally distilled. Then the less volatile fractions are subjected to further treatment called cracking
Cracking of petroleum
In the early days, the petroleum fractions in highest demands were the lubricating oils and paraffin wax Since the invention of the motor car, the demand for the petrol fraction has been increasing.
Generally, for most crude oils, the petrol fraction makes up only 20 to 40% of the distillates, and out of these, only a small portion is suitable for use as a furt in the engines of motor cars. Due to the high demand for petrol, other less useful fractions such as kerosene and gas oil are converted into petrol by a process called the cracking of petroleum Cracking means breaking down or decomposition of a compound by the action of heat alone. This process involves splitting larger molecules into smaller molecules by subjecting them to high temperatures and pressures, usually in the presence of a catalyst. Cracking is the process by which a heavier hydrocarbon molecule is splitted into two or more lighter hydrocarbon molecule.
In thermal cracking of petroleum, the less volatile crude oil fractions are simply passed through a chamber heated to a very high temperature (above 1000 °C). Long chain alkanes are converted into smaller chain alkanes, alkenes and hydrogen, with ethene, C₂H₄, being the predominant alkene present. Depending on the reaction conditions, cracking can take place at any point along the main carbon skeleton of the alkane to give shorter chain alkanes or alkenes. The cracking of decane is shown.
NOTE: The products given are only some of the many possible products formed during the cracking of decane.
Thermal cracking is not particularly efficient because there is post control over the cracking pattern to yield abanos of desirable chain length. This is due to the fact that the carbon-to-carbon bonds of the alkanes in the heavy oil fractions are about the strength
ORGANIC CHEMISTRY II
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
METALS AND COMPOUNDS
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.